Nonivamide
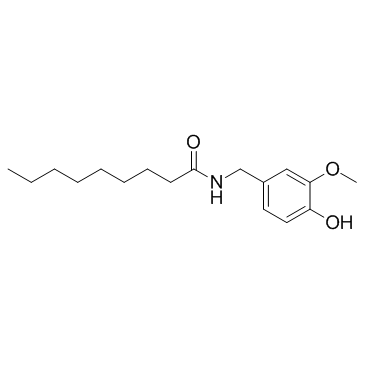
Nonivamide structure
|
Common Name | Nonivamide | ||
|---|---|---|---|---|
| CAS Number | 2444-46-4 | Molecular Weight | 293.401 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 450.4±55.0 °C at 760 mmHg | |
| Molecular Formula | C17H27NO3 | Melting Point | 54°C | |
| MSDS | Chinese USA | Flash Point | 226.2±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of NonivamideNonivamide is a agonist, which exhibits 4d-EC50 value of 5.1 mg/L in static toxicity tests. |
| Name | Nonivamide |
|---|---|
| Synonym | More Synonyms |
| Description | Nonivamide is a agonist, which exhibits 4d-EC50 value of 5.1 mg/L in static toxicity tests. |
|---|---|
| Related Catalog | |
| Target |
TRPV1[1] |
| In Vitro | Nonivamide, a synthetic derivate of natural capsaicin, has an effective antifouling activity. Capsaicin exhibits 4d-EC50 values of 5.5±0.5 mg/L, 23±2 mg/L, 6.9±0.2 mg/L, and 15.6±0.4 mg/L in static toxicity tests conducted using Pseudomonas putida, Lake Erie bacteria, Vibrio natriegens, and Vibrio parahaemolyticus, respectively. A significant growth inhibitory effect (p<0.01) is observed in the group treated with 1 mg/L of Nonivamide for 4 d, and the EC50 value (4 d-EC50) is 5.1 mg/L[1]. Nonivamide treatment causes calcium release from the ER and altered the transcription of growth arrest- and DNA damage-inducible transcript 3 (GADD153), GADD45α, GRP78/BiP, ATF3, CCND1, and CCNG2) in a manner comparable with prototypical ER stress-inducing agents. ER calcium flux is evaluated by pretreating cells with 2.5 μM thapsigargin for 5 min followed by addition of 2.5 μM Nonivamide. Treatment of TRPV1-overexpressing cells with 2.5 μM Nonivamide produces marked increases in cytosolic calcium due to release of calcium from ER stores. Treatment of TRPV1-overexpressing cells with 1 μM Nonivamide causes an approximate 50% loss in cell viability after a 24-h period. BEAS-2B cells treated with 100 and 200 μM Nonivamide also exhibits a shift in the relative amount of EIF2α-P and an increase in the expression of GADD153 mRNA and protein[2]. Treatment with Nonivamide reduces lipid accumulation to a similar extent as CAP; the effects are not different from the effects after CAP treatment at any of the tested concentrations. Compared to untreated control cells, treatment with Nonivamide decreases lipid accumulation by 5.34±1.03% (P<0.05) at 0.01 µM up to 10.4±2.47% (P<0.001) at 1 µM[3]. |
| Cell Assay | In the MTT assay, the reduction of yellow tetrazolium salt MTT to a purple formazan by mitochondrial and ER enzymes is used as a measure for cell viability. Cells are seeded in 96‐well plates and treated with 1 nM-10 µM CAP or Nonivamide with or without addition of 25-100 µM BCH or the corresponding ethanol concentration (0.1-0.2% (v/v), solvent control) for 12 days after initiation of differentiation. Cell culture media is exchanged every second day. On Day 12, 100 µL of the MTT working reagent (0.83 mg/mL MTT diluted in PBS/serum-free media (1:5)), is added to each well, and cells are incubated at 37°C for approximately 15 min. The MTT working solution is removed and the purple formazan formed during incubation is dissolved in 150 µL DMSO per well. Absorbance is measured at 550 nm with 690 nm as reference wavelength using multiwell plate reader. The number of metabolically active cells is calculated relative to untreated control cells or the corresponding solvent control (100%)[3]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 450.4±55.0 °C at 760 mmHg |
| Melting Point | 54°C |
| Molecular Formula | C17H27NO3 |
| Molecular Weight | 293.401 |
| Flash Point | 226.2±31.5 °C |
| Exact Mass | 293.199097 |
| PSA | 58.56000 |
| LogP | 4.38 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.509 |
| InChIKey | RGOVYLWUIBMPGK-UHFFFAOYSA-N |
| SMILES | CCCCCCCCC(=O)NCc1ccc(O)c(OC)c1 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | methanol: 100 mg/mL, clear to slightly hazy |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H317-H319-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | S26-S45 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | RA5998000 |
| Hazard Class | 6.1 |
| HS Code | 2924299090 |
| Precursor 9 | |
|---|---|
| DownStream 4 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Identification and mechanosensitivity of viscerofugal neurons.
Neuroscience 225 , 118-29, (2012) Enteric viscerofugal neurons are interneurons with cell bodies in the gut wall; they project to prevertebral ganglia where they provide excitatory synaptic drive to sympathetic neurons which control i... |
|
|
Nasal allergen deposition leads to conjunctival mast cell degranulation in allergic rhinoconjunctivitis.
Am. J. Rhinol. Allergy 28(4) , 290-6, (2014) The naso-ocular interaction in allergic rhinoconjunctivitis is well recognized from epidemiological, clinical, and experimental observations. The precise mechanisms remain incompletely understood. A n... |
| N-[(4-hydroxy-3-methoxy-phenyl)methyl]nonanamide |
| Nonanimidic acid, N-[(4-hydroxy-3-methoxyphenyl)methyl]-, (1Z)- |
| N-vanillyl-nonanamide |
| Vanillyl pelargonic amide |
| (N-(4-Hydroxy-3-methoxybenzyl)nonanamide |
| Nonivamide |
| Vanillyl-N-nonylamide |
| Pseudocapsaicin |
| Capsaicin (Synthetic) |
| N-VANILLYLNONANOAMIDE |
| Nonanamide, N-vanillyl- |
| Pelargonyl vanillylamide |
| N-Nonylvanylamide |
| NonvaMide |
| Capsaicin std. |
| nonanoic acid vanillylamide |
| EINECS 219-484-1 |
| N-(4-Hydroxy-3-methoxybenzyl)nonanamide |
| Capsaicinoid |
| N-Pelargonic Acid Vanillylamide |
| MFCD00017286 |
| Capsaicin synthetic |
| Pelargonic acid vanillylamide |
| NONYLVANYLAMIDE |
| N-Pelargonylvanillylamide |
| N-Vanillylnonanamide |
| Vanillyl n-nonoylamide |
| nonylic acid vanillylamide |
| PAVA |
| NON-4-ENE |
| (1Z)-N-(4-Hydroxy-3-methoxybenzyl)nonanimidic acid |
| Nonanamide, N-[(4-hydroxy-3-methoxyphenyl)methyl]- |
| N-Vanillylpelargonamide |
| N-Vanillyl nonan amide |
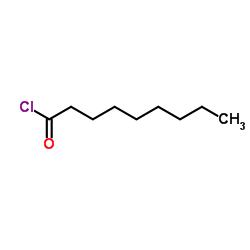 CAS#:764-85-2
CAS#:764-85-2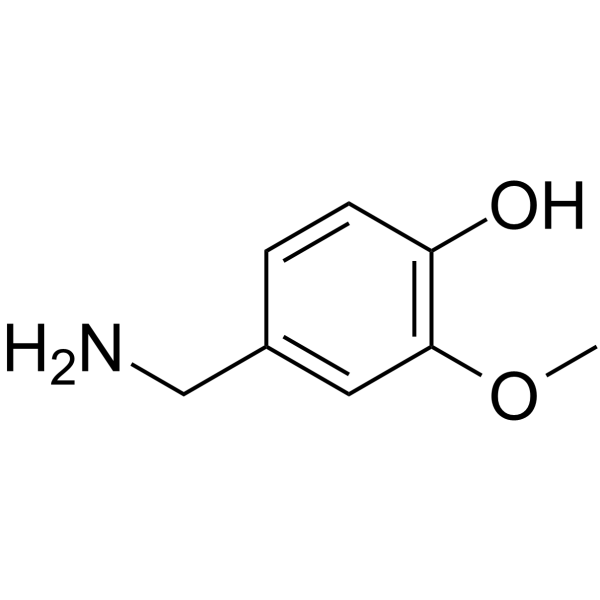 CAS#:1196-92-5
CAS#:1196-92-5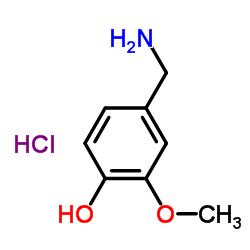 CAS#:7149-10-2
CAS#:7149-10-2 CAS#:112-05-0
CAS#:112-05-0 CAS#:1120-07-6
CAS#:1120-07-6 CAS#:4421-08-3
CAS#:4421-08-3 CAS#:90-05-1
CAS#:90-05-1 CAS#:102811-97-2
CAS#:102811-97-2 CAS#:2426-87-1
CAS#:2426-87-1![sodium,2-[2-methoxy-4-[(nonanoylamino)methyl]phenoxy]acetate structure](https://image.chemsrc.com/caspic/270/146690-24-6.png) CAS#:146690-24-6
CAS#:146690-24-6![N-[[4-(2-hydroxyethoxy)-3-methoxyphenyl]methyl]nonanamide structure](https://image.chemsrc.com/caspic/497/143827-59-2.png) CAS#:143827-59-2
CAS#:143827-59-2 CAS#:1731-84-6
CAS#:1731-84-6
