(S)-Higenamine
Modify Date: 2025-08-27 13:20:57
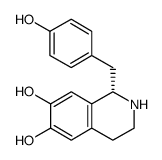
(S)-Higenamine structure
|
Common Name | (S)-Higenamine | ||
|---|---|---|---|---|
| CAS Number | 22672-77-1 | Molecular Weight | 271.31100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H17NO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (S)-Higenamine(S)-Higenamine ((S)-Norcoclaurine) is the entry compound in benzylisoquinoline alkaloid biosynthesis. (S)-Higenamine is produced by the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) by norcoclaurine synthase (NCS)[1]. |
| Name | (S)-norcoclaurine |
|---|
| Description | (S)-Higenamine ((S)-Norcoclaurine) is the entry compound in benzylisoquinoline alkaloid biosynthesis. (S)-Higenamine is produced by the condensation of dopamine and 4-hydroxyphenylacetaldehyde (4-HPAA) by norcoclaurine synthase (NCS)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | The biosynthetic pathway leading to benzylisoquinoline alkaloids originates from the enzyme-catalyzed condensation of dopamine and 4-hydrophenylacetaldehyde to yield (S)-norcoclaurine. Both substrates are secondary metabolites derived from the decarboxylation/hydroxylation/deamination of tyrosine[1]. |
| References |
| Molecular Formula | C16H17NO3 |
|---|---|
| Molecular Weight | 271.31100 |
| Exact Mass | 271.12100 |
| PSA | 72.72000 |
| LogP | 2.56170 |