17beta-estradiol-2,4,16,16,17-d5
Modify Date: 2025-08-26 18:15:01
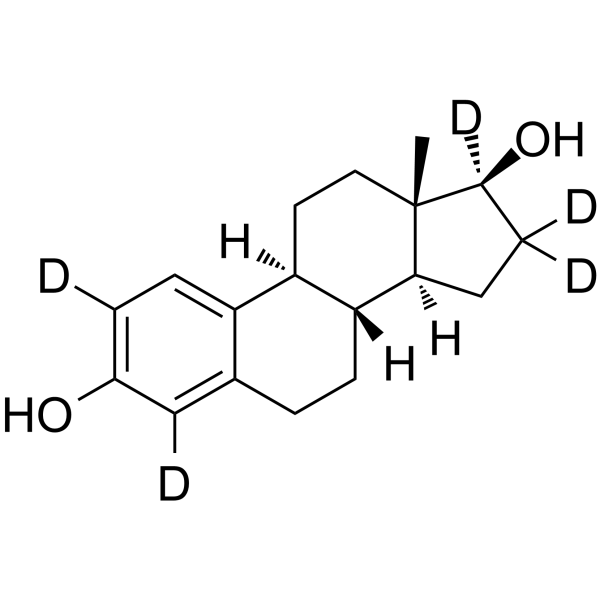
17beta-estradiol-2,4,16,16,17-d5 structure
|
Common Name | 17beta-estradiol-2,4,16,16,17-d5 | ||
|---|---|---|---|---|
| CAS Number | 221093-45-4 | Molecular Weight | 277.41 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 445.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C18H19D5O2 | Melting Point | 178-179ºC(lit.) | |
| MSDS | USA | Flash Point | 209.6±23.3 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of 17beta-estradiol-2,4,16,16,17-d5Estradiol-d5 is deuterium labeled Estradiol. Estradiol is a steroid sex hormone vital to the maintenance of fertility and secondary sexual characteristics in females. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway[1][2][3]. |
| Name | (8R,9S,13S,14S,17S)-2,4,16,16,17-pentadeuterio-13-methyl-6,7,8,9,11,12,14,15-octahydrocyclopenta[a]phenanthrene-3,17-diol |
|---|---|
| Synonym | More Synonyms |
| Description | Estradiol-d5 is deuterium labeled Estradiol. Estradiol is a steroid sex hormone vital to the maintenance of fertility and secondary sexual characteristics in females. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 445.9±45.0 °C at 760 mmHg |
| Melting Point | 178-179ºC(lit.) |
| Molecular Formula | C18H19D5O2 |
| Molecular Weight | 277.41 |
| Flash Point | 209.6±23.3 °C |
| Exact Mass | 277.209015 |
| PSA | 40.46000 |
| LogP | 4.13 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.599 |
| InChIKey | VOXZDWNPVJITMN-MOKSCAJFSA-N |
| SMILES | CC12CCC3c4ccc(O)cc4CCC3C1CCC2O |
| Storage condition | ?20°C |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H350 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R45 |
| Safety Phrases | 53-45 |
| RIDADR | UN 1648 3 / PGII |
|
~95% 
17beta-estradio... CAS#:221093-45-4 |
| Literature: Kiuru, Paula S.; Waehaelae, Kristiina Steroids, 2006 , vol. 71, # 1 p. 54 - 60 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| 17|A-Estradiol-2,4,16,16,17-d5 |
| (17β)-(2,4,16,16,17-H)Estra-1,3,5(10)-triene-3,17-diol |
| Estra-1,3,5(10)-triene-2,4,16,16,17-d-3,17-diol, (17β)- |