UB 165 fumarate
Modify Date: 2025-08-26 13:12:32
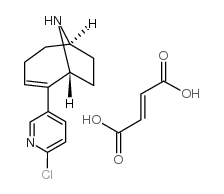
UB 165 fumarate structure
|
Common Name | UB 165 fumarate | ||
|---|---|---|---|---|
| CAS Number | 200432-86-6 | Molecular Weight | 350.79700 | |
| Density | N/A | Boiling Point | 597.4ºC at 760mmHg | |
| Molecular Formula | C17H19ClN2O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 315.1ºC | |
Use of UB 165 fumarateUB-165 is a nAChR agonist, being a full agonist of the α3β2 isoform and a partial agonist of the α4β2* isoform, with a Ki value of 0.27 nM for [3H]-nicotine binding in rat brain[1]. |
| Name | UB 165 fumarate,2-(6-Chloro-3-pyridinyl)-9-azabicyclo[4.2.1]non-2-enefumarate |
|---|---|
| Synonym | More Synonyms |
| Description | UB-165 is a nAChR agonist, being a full agonist of the α3β2 isoform and a partial agonist of the α4β2* isoform, with a Ki value of 0.27 nM for [3H]-nicotine binding in rat brain[1]. |
|---|---|
| Related Catalog | |
| In Vitro | UB-165 刺激 [3H]-多巴胺从纹状体突触释放,EC50 值为 88 nM[1]。 |
| References |
| Boiling Point | 597.4ºC at 760mmHg |
|---|---|
| Molecular Formula | C17H19ClN2O4 |
| Molecular Weight | 350.79700 |
| Flash Point | 315.1ºC |
| Exact Mass | 350.10300 |
| PSA | 99.52000 |
| LogP | 3.07340 |
| Compound 401 |
| UB 165 FUMARATE |