AR-R17779 hydrochloride
Modify Date: 2025-08-24 19:47:56
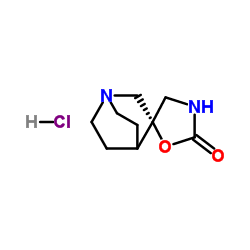
AR-R17779 hydrochloride structure
|
Common Name | AR-R17779 hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 178419-42-6 | Molecular Weight | 218.681 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C9H15ClN2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of AR-R17779 hydrochlorideAR-R17779 hydrochloride is a potent and selective full agonist of nAChR, with Kis of 92 and 16000 nM for α7 and α4β2 subtype, respectively. AR-R17779 hydrochloride can improve learning and memory in rats. AR-R17779 hydrochloride also has anxiolytic activity. AR-R17779 hydrochloride can reduce inflammation by activating antiinflammatory cholinergic (vagal) pathways[1][2][4]. |
| Name | (2S)-2'H-Spiro[4-azabicyclo[2.2.2]octane-2,5'-[1,3]oxazolidin]-2'-one hydrochloride (1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | AR-R17779 hydrochloride is a potent and selective full agonist of nAChR, with Kis of 92 and 16000 nM for α7 and α4β2 subtype, respectively. AR-R17779 hydrochloride can improve learning and memory in rats. AR-R17779 hydrochloride also has anxiolytic activity. AR-R17779 hydrochloride can reduce inflammation by activating antiinflammatory cholinergic (vagal) pathways[1][2][4]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 92 nM (α7-nAChR)[1] |
| In Vitro | AR-R17779 is 5-fold more potent and 35000-fold more selective than (-)-nicotine for the α7 nicotinic receptor[1]. AR-R17779 (200 nM; 24 h) inhibits the LPS-induced TNF production in macrophages[4]. |
| In Vivo | AR-R17779 (1-5 mg/kg; i.p. twice a day for 7 d) ameliorates arthritis, reduces synovial inflammation, delays onset of disease and protects against joint destruction[3]. AR-R17779 (1-10 mg/kg; s.c. for 3 weeks) improves learning in two radial-arm maze tasks and reverses working memory impairment caused by fimbria-fornix sections in rats[2]. Animal Model: Male DBA/1 mice (8-10 weeks) were subjected to unilateral cervical vagotomy or sham surgery, after which arthritis was induced with type II collagen[3] Dosage: 1, 2.5, 5 mg/kg Administration: I.p. twice daily from day 20 until day 26 Result: Ameliorated arthritis and delayed onset of disease. Reduced erosive disease, cartilage degradation and synovial inflammation. Reduced TNFα levels in plasma and synovial tissue. |
| References |
| Molecular Formula | C9H15ClN2O2 |
|---|---|
| Molecular Weight | 218.681 |
| Exact Mass | 218.082199 |
| Hazard Codes | C |
|---|
| Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidin]-2'-one, (3S)-, hydrochloride (1:1) |
| (2S)-2'H-Spiro[4-azabicyclo[2.2.2]octane-2,5'-[1,3]oxazolidin]-2'-one hydrochloride (1:1) |