5-Hexenoic acid

5-Hexenoic acid structure
|
Common Name | 5-Hexenoic acid | ||
|---|---|---|---|---|
| CAS Number | 1577-22-6 | Molecular Weight | 114.142 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 202.6±9.0 °C at 760 mmHg | |
| Molecular Formula | C6H10O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 100.1±13.9 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | 5-hexenoic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 202.6±9.0 °C at 760 mmHg |
| Molecular Formula | C6H10O2 |
| Molecular Weight | 114.142 |
| Flash Point | 100.1±13.9 °C |
| Exact Mass | 114.068077 |
| PSA | 37.30000 |
| LogP | 1.36 |
| Vapour Pressure | 0.1±0.8 mmHg at 25°C |
| Index of Refraction | 1.444 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45-S27 |
| RIDADR | 3265 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2916190090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916190090 |
|---|---|
| Summary | 2916190090 unsaturated acyclic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:6.5%。general tariff:30.0% |
|
Production of functionalized polyhydroxyalkanoates by genetically modified Methylobacterium extorquens strains.
Microb. Cell Fact. 9 , 70, (2010) Methylotrophic (methanol-utilizing) bacteria offer great potential as cell factories in the production of numerous products from biomass-derived methanol. Bio-methanol is essentially a non-food substr... |
|
|
Tertiary aminourea-catalyzed enantioselective iodolactonization.
Angew. Chem. Int. Ed. Engl. 49(40) , 7332-5, (2010)
|
|
|
Synthesis of fluoroalkyl-d-lactones from polyfluoroalkyl iodides and 5-hexenoic acids. Fang X, et al.
J. Fluor. Chem. 129(4) , 280-285., (2008)
|
| Hex-5-enoic acid |
| 5-Hexenoic acid |
| MFCD00046558 |
 CAS#:821-41-0
CAS#:821-41-0 CAS#:7531-60-4
CAS#:7531-60-4 CAS#:63831-51-6
CAS#:63831-51-6 CAS#:124-38-9
CAS#:124-38-9 CAS#:1119-51-3
CAS#:1119-51-3 CAS#:75142-64-2
CAS#:75142-64-2 CAS#:129266-34-8
CAS#:129266-34-8 CAS#:108-94-1
CAS#:108-94-1 CAS#:4812-17-3
CAS#:4812-17-3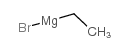 CAS#:925-90-6
CAS#:925-90-6 CAS#:21963-38-2
CAS#:21963-38-2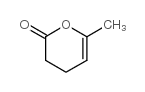 CAS#:3740-59-8
CAS#:3740-59-8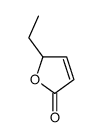 CAS#:2407-43-4
CAS#:2407-43-4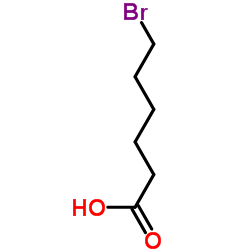 CAS#:4224-70-8
CAS#:4224-70-8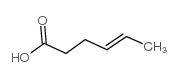 CAS#:35194-36-6
CAS#:35194-36-6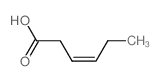 CAS#:4219-24-3
CAS#:4219-24-3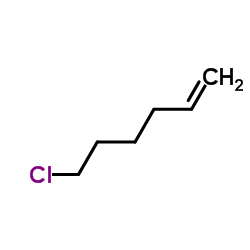 CAS#:928-89-2
CAS#:928-89-2 CAS#:110-94-1
CAS#:110-94-1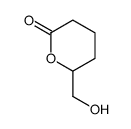 CAS#:81683-96-7
CAS#:81683-96-7