SNC80
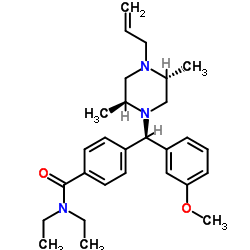
SNC80 structure
|
Common Name | SNC80 | ||
|---|---|---|---|---|
| CAS Number | 156727-74-1 | Molecular Weight | 449.628 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 564.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C28H39N3O2 | Melting Point | 122-123ºC | |
| MSDS | Chinese USA | Flash Point | 295.4±30.1 °C | |
Use of SNC80SNC80 (NIH 10815) is a potent, highly selective and non-peptide δ-opioid receptor agonist with a Ki of 1.78 nM and an IC50 of 2.73 nM. SNC80 also selectively activates μ-δ heteromer in HEK293 cells with an EC50 of 52.8 nM. SNC80 shows antinociceptive, antihyperalgesic and antidepressant‐like effects. SNC80 has the potential for multiple headache disorders treatment[1][2][3][4][5][6]. |
| Name | 4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | SNC80 (NIH 10815) is a potent, highly selective and non-peptide δ-opioid receptor agonist with a Ki of 1.78 nM and an IC50 of 2.73 nM. SNC80 also selectively activates μ-δ heteromer in HEK293 cells with an EC50 of 52.8 nM. SNC80 shows antinociceptive, antihyperalgesic and antidepressant‐like effects. SNC80 has the potential for multiple headache disorders treatment[1][2][3][4][5][6]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 2.73 nM (δ-opioid receptor), 5457 nM (μ-opioid receptor)[3] Ki: 1.78 nM (δ-opioid receptor), 881.5 nM (μ-opioid receptor) and 441.8 nM (κ-opioid receptor)[2] |
| In Vitro | SNC80 selectively activates μ-δ heteromer in HEK293 cells with an EC50 of 52.8 nM. SNC80 exhibits substantially greater activity in cells coexpressing μ- and δ-opioid receptors than in cells either singly expressing δ-opioid receptors or coexpressing δ- and κ-opioid receptors[4]. |
| In Vivo | SNC80 (10 mg/kg; intraperitoneal injection; once; C57BL6/J mice) treatment significantly attenuated this allodynia caused by overuse of Sumatriptan[1]. Animal Model: Male and female C57BL6/J mice (20-30g) injected with Sumatriptan[1] Dosage: 10 mg/kg Administration: Intraperitoneal injection; once Result: Significantly attenuated allodynia. |
| References |
[5]. Vicente-Sanchez A, et al. The delta opioid receptor tool box. Neuroscience. 2016 Dec 3;338:145-159. |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 564.8±50.0 °C at 760 mmHg |
| Melting Point | 122-123ºC |
| Molecular Formula | C28H39N3O2 |
| Molecular Weight | 449.628 |
| Flash Point | 295.4±30.1 °C |
| Exact Mass | 449.304230 |
| PSA | 36.02000 |
| LogP | 3.37 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.545 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
|
PKA and ERK1/2 are involved in dopamine D₁ receptor-induced heterologous desensitization of the δ opioid receptor.
Life Sci. 92(23) , 1101-9, (2013) Chronic administration of cocaine attenuates delta opioid receptor (DOPR) signaling in the striatum and the desensitization is mediated by the indirect actions of cocaine on dopamine D1 receptors (D1R... |
|
|
Novel screening assay for the selective detection of G-protein-coupled receptor heteromer signaling.
J. Pharmacol. Exp. Ther. 344(1) , 179-88, (2013) Drugs targeting G-protein-coupled receptors (GPCRs) make up more than 25% of all prescribed medicines. The ability of GPCRs to form heteromers with unique signaling properties suggests an entirely new... |
|
|
Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety.
J. Pharmacol. Exp. Ther. 335(1) , 133-9, (2010) Alcoholism and anxiety disorders have a huge impact on society and afflict 17.6 million and 40 million people in the United States, respectively. A strong comorbidity exists between alcoholism and anx... |
| (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide |
| 4-[(R)-[(2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide |
| SNC80 |
| Benzamide, 4-[(R)-[(2S,5R)-2,5-dimethyl-4-(2-propen-1-yl)-1-piperazinyl](3-methoxyphenyl)methyl]-N,N-diethyl- |
| 4-[(R)-[(2S,5R)-4-Allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide |
| MFCD00672673 |
| 2-Pyridylethylamine dihydrochloride |
 CAS#:58287-77-7
CAS#:58287-77-7 CAS#:36282-40-3
CAS#:36282-40-3 CAS#:155836-78-5
CAS#:155836-78-5 CAS#:186094-10-0
CAS#:186094-10-0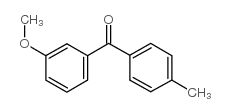 CAS#:82520-37-4
CAS#:82520-37-4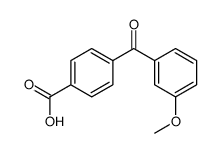 CAS#:156727-76-3
CAS#:156727-76-3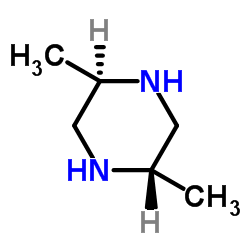 CAS#:2815-34-1
CAS#:2815-34-1 CAS#:619-66-9
CAS#:619-66-9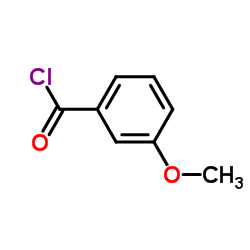 CAS#:1711-05-3
CAS#:1711-05-3