Diclofenac sodium
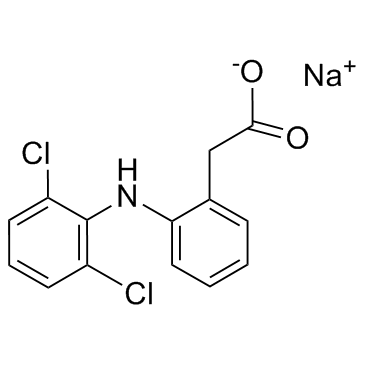
Diclofenac sodium structure
|
Common Name | Diclofenac sodium | ||
|---|---|---|---|---|
| CAS Number | 15307-79-6 | Molecular Weight | 318.130 | |
| Density | N/A | Boiling Point | 412ºC at 760 mmHg | |
| Molecular Formula | C14H10Cl2NNaO2 | Melting Point | 288-290°C | |
| MSDS | Chinese USA | Flash Point | 203ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Diclofenac sodiumDiclofenac Sodium is a potent and nonselective anti-inflammatory agent, acts as a COX inhibitor, with IC50s of 4 nM, 1.3 nM for human COX-1 and COX-2 in CHO cells, and 5.1, 0.84 μM for ovine COX-1 and COX-2, respectively. |
| Name | diclofenac sodium |
|---|---|
| Synonym | More Synonyms |
| Description | Diclofenac Sodium is a potent and nonselective anti-inflammatory agent, acts as a COX inhibitor, with IC50s of 4 nM, 1.3 nM for human COX-1 and COX-2 in CHO cells, and 5.1, 0.84 μM for ovine COX-1 and COX-2, respectively. |
|---|---|
| Related Catalog | |
| Target |
Human COX-2:1.3 nM (IC50, in CHO cells) Human COX-1:4 nM (IC50, in CHO cells) Ovine COX-2:0.84 μM (IC50) Ovine COX-1:5.1 μM (IC50) |
| In Vitro | Diclofenac Sodium is a potent COX inhibitor, with IC50s of 4 nM and 1.3 nM for human COX-1 and COX-2 in the CHO cells, respectively. Diclofenac effectively blocks COX-1 mediated prostanoid production from U937 cell microsomes, with an IC50 of 7 ± 3 nM[1]. Diclofenac Sodium exihibits inhibition on COX-1 and COX-2 enzyme with IC50s of 5.1 and 0.84 μM, respectively[2]. |
| In Vivo | Diclofenac (3 mg/kg, b.i.d., for 5 days) significantly increases faecal 51Cr excretion in rats, and such effect is also observed in squirrel monkeys after administrated of 1 mg/kg twice daily for 4 days[1]. Diclofenac (10 mg/kg) shows anti-inflammatory activity in mice[2]. Diclofenac (10 mg/kg) decreases oxidized low-densitylipoprotein (Ox-LDL), but shows no effects on the kinetics parameters of catalase and glutathione peroxidase via intramuscularly injection into rats[3]. |
| Animal Admin | Rats[1] Male Sprague-Dawley rats (150 ± 200 g) are dosed orally with Diclofenac either once (acute dosing) or twice daily for 5 days (chronic dosing). A plasma sample is obtained 1 h after the morning dose on day 4 for measurement of Diclofenac concentration. Immediately after the administration of the last dose on day 5, the rats are injected via a tail vein with 0.5 mL of 51Cr-labelled red blood cells from a donor rat after incubation with sodium 51chromate. The rats are placed individually in metabolism cages with food and water ad libitum. Faeces are collected for a 48 h period and 51Cr faecal excretion is calculated as a % of total injected dose (20 mCi per animal)[1]. Squirrel monkeys[1] Squirrel monkeys (Saimiri sciureus; 0.8 ± 1.4 kg) are dosed orally with Diclofenac twice daily for 1 ± 5 days. One hour after administration of the last dose, 51CrCl3 in sterile saline (1 mL/kg, 4 ± 5 mCi per animal) is injected via a saphenous vein and plasma samples are obtained for measurement of Diclofenac concentration. The monkeys are then housed individually in metabolism cages. Faeces are collected for a 24 h period and 51Cr faecal excretion is calculated as a % of total injected dose[1]. |
| References |
| Boiling Point | 412ºC at 760 mmHg |
|---|---|
| Melting Point | 288-290°C |
| Molecular Formula | C14H10Cl2NNaO2 |
| Molecular Weight | 318.130 |
| Flash Point | 203ºC |
| Exact Mass | 316.998627 |
| PSA | 52.16000 |
| LogP | 3.10240 |
| Storage condition | -20°C Freezer |
| Stability | Stable. |
| Water Solubility | H2O: 50 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | S22-S36/37-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AG6330000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2922499990 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Diclofenac toxicity in human intestine ex vivo is not related to the formation of intestinal metabolites.
Arch. Toxicol. 89(1) , 107-19, (2015) The use of diclofenac (DCF), a nonsteroidal anti-inflammatory drug, is associated with a high prevalence of gastrointestinal side effects. In vivo studies in rodents suggested that reactive metabolite... |
|
|
Multifunctional medicated lyophilised wafer dressing for effective chronic wound healing.
J. Pharm. Sci. 103(6) , 1720-33, (2014) Wafers combining weight ratios of Polyox with carrageenan (75/25) or sodium alginate (50/50) containing streptomycin and diclofenac were prepared to improve chronic wound healing. Gels were freeze-dri... |
|
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of... |
| Novapirina |
| Dicloreum |
| (o-(2,6-dichloroanilino)phenyl)acetic acid sodium salt |
| (o-(2,6-dichloroanilino)phenyl)acetic acid monosodium salt |
| 2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid s |
| MFCD00082251 |
| Naclof |
| Benzeneacetic acid, 2-[(2,6-dichlorophenyl)amino]-, sodium salt (1:1) |
| VALETAN |
| Ecofenac |
| EINECS 239-346-4 |
| Sodium 2-(2,6-Dichloroanilino)phenylacetate |
| 2-[(2,6-Dichlorophenyl)amino]benzeneacetic Acid Monosodium Salt |
| Xenid |
| benzeneacetic acid, 2-[(2,6-dichlorophenyl)amino]-, monosodium salt |
| KROPLEX |
| Sodium {2-[(2,6-dichlorophenyl)amino]phenyl}acetate |
| 2-(2,6-Dichloroanilino)phenylacetic Acid Sodium Salt |
| Primofenac |
| kriplex |
| Voltaren |
| Diacron |
| Benfofen |
| Diclofenac Sodium Salt |
| Rhumalgan |
| Voldal |
| Effekton |
| Diclofenac sodium |
| 2-[(2,6-Dichlorophenyl)amino]benzeneacetic acid sodium salt |
| Duravolten |
| Neriodin |
| Prophenatin |
| Aceclofenac Impurity 1 |
| Diclofenac (Sodium) |
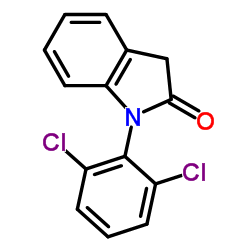 CAS#:15362-40-0
CAS#:15362-40-0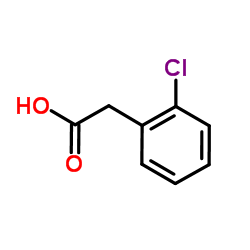 CAS#:2444-36-2
CAS#:2444-36-2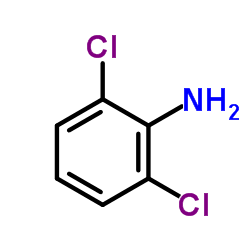 CAS#:608-31-1
CAS#:608-31-1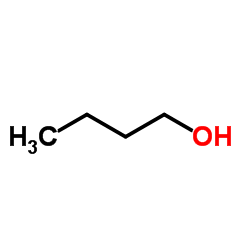 CAS#:71-36-3
CAS#:71-36-3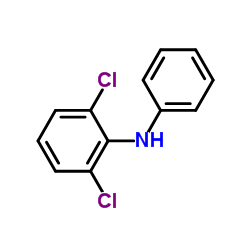 CAS#:15307-93-4
CAS#:15307-93-4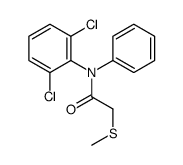 CAS#:83281-93-0
CAS#:83281-93-0 CAS#:83281-96-3
CAS#:83281-96-3 CAS#:66156-75-0
CAS#:66156-75-0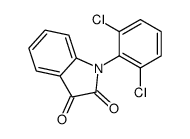 CAS#:24542-74-3
CAS#:24542-74-3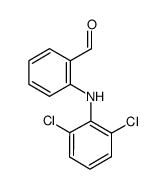 CAS#:22121-58-0
CAS#:22121-58-0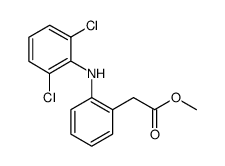 CAS#:15307-78-5
CAS#:15307-78-5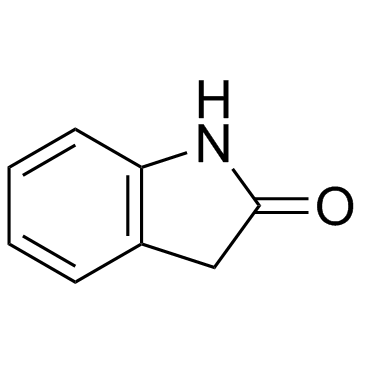 CAS#:59-48-3
CAS#:59-48-3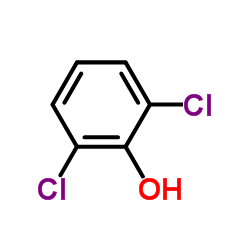 CAS#:87-65-0
CAS#:87-65-0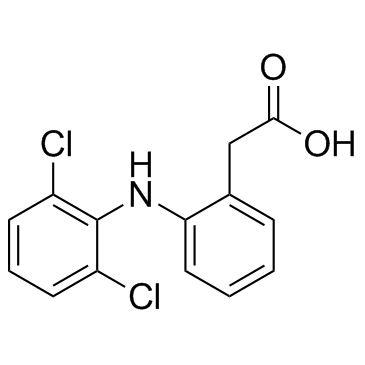 CAS#:15307-86-5
CAS#:15307-86-5 CAS#:27204-57-5
CAS#:27204-57-5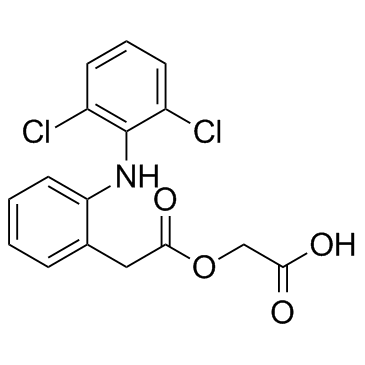 CAS#:89796-99-6
CAS#:89796-99-6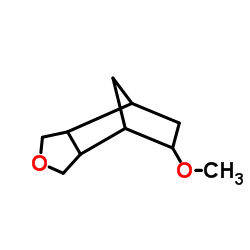 CAS#:70172-33-7
CAS#:70172-33-7
