Gold(1+) chloride-triphenylphosphine (1:1:1)
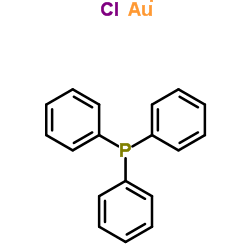
Gold(1+) chloride-triphenylphosphine (1:1:1) structure
|
Common Name | Gold(1+) chloride-triphenylphosphine (1:1:1) | ||
|---|---|---|---|---|
| CAS Number | 14243-64-2 | Molecular Weight | 494.71 | |
| Density | N/A | Boiling Point | 360ºC at 760 mmHg | |
| Molecular Formula | C18H15AuClP | Melting Point | 248-249°C | |
| MSDS | Chinese USA | Flash Point | 181.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Gold(1+) chloride-triphenylphosphine (1:1:1)Triphenylphosphinechlorogold is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Chloro(triphenylphosphine)gold(I) |
|---|---|
| Synonym | More Synonyms |
| Description | Triphenylphosphinechlorogold is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Boiling Point | 360ºC at 760 mmHg |
|---|---|
| Melting Point | 248-249°C |
| Molecular Formula | C18H15AuClP |
| Molecular Weight | 494.71 |
| Flash Point | 181.7ºC |
| Exact Mass | 494.026489 |
| PSA | 13.59000 |
| LogP | 4.13180 |
| Vapour Pressure | 4.74E-05mmHg at 25°C |
| Storage condition | Store under Nitrogen |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39-S37/39 |
| RIDADR | NONH for all modes of transport |
| Precursor 8 | |
|---|---|
| DownStream 9 | |
|
A mass spectrometric investigation of the binding of gold antiarthritic agents and the metabolite [Au(CN)2]- to human serum albumin.
J. Biol. Inorg. Chem. 11(5) , 559-70, (2006) Electrospray ionisation (ESI) mass spectrometry was used to examine the reactions of the clinically used antiarthritic agent [Au(S2O3)2]3-, and AuPEt3Cl, a derivative of another clinically used agent ... |
|
|
Development of a gold-multifaceted catalysis approach to the synthesis of highly substituted pyrroles: mechanistic insights via Huisgen cycloaddition studies.
J. Org. Chem. 78(3) , 920-34, (2013) A novel gold-catalyzed method for the regioselective synthesis of highly substituted pyrroles directly from oximes and alkynes was developed via independent optimization of two key steps of the proces... |
|
|
A powerful chiral counterion strategy for asymmetric transition metal catalysis.
Science 317 , 496-499, (2007) Traditionally, transition metal-catalyzed enantioselective transformations rely on chiral ligands tightly bound to the metal to induce asymmetric product distributions. Here we report high enantiosele... |
| Phosphine, triphenyl-, gold(1+) salt, hydrochloride (1:1:1) |
| Gold(1+) chloride - triphenylphosphine (1:1:1) |
| (Triphenylphosphine)gold(I) Chloride |
| EINECS 238-117-6 |
| Chloro(triphenylphosphine)gold |
| (Ph3P)AuCl (Triphenylphosphinegold(I) chloride |
| MFCD00009588 |
| chlorogold,triphenylphosphane |
 CAS#:39929-21-0
CAS#:39929-21-0 CAS#:603-35-0
CAS#:603-35-0 CAS#:616-47-7
CAS#:616-47-7![cis-[AuIII(C6F5)Cl2(PPh3)] Structure](https://image.chemsrc.com/caspic/066/37822-98-3.png) CAS#:37822-98-3
CAS#:37822-98-3![Gold, [1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]methanesulfonamidato-κN](triphenylphosphine)- Structure](https://image.chemsrc.com/caspic/312/866395-16-6.png) CAS#:866395-16-6
CAS#:866395-16-6 CAS#:29892-37-3
CAS#:29892-37-3 CAS#:1582-24-7
CAS#:1582-24-7![[AuCl(2,2'-thiodiethanol)] Structure](https://image.chemsrc.com/caspic/090/69462-32-4.png) CAS#:69462-32-4
CAS#:69462-32-4![gold,2-[4-[2-(4-nitrophenyl)ethynyl]phenyl]ethynyl-triphenylphosphanium structure](https://image.chemsrc.com/caspic/253/185119-04-4.png) CAS#:185119-04-4
CAS#:185119-04-4 CAS#:20224-83-3
CAS#:20224-83-3 CAS#:18024-34-5
CAS#:18024-34-5 CAS#:75-36-5
CAS#:75-36-5 CAS#:24228-71-5
CAS#:24228-71-5 CAS#:12092-47-6
CAS#:12092-47-6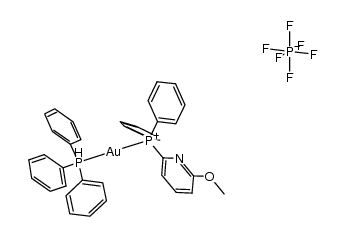 CAS#:143890-25-9
CAS#:143890-25-9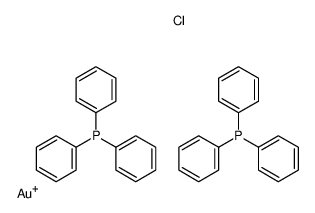 CAS#:14853-97-5
CAS#:14853-97-5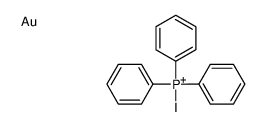 CAS#:21209-78-9
CAS#:21209-78-9
