N-Allylnormetazocine hydrochloride
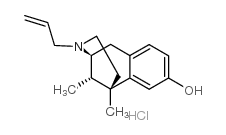
N-Allylnormetazocine hydrochloride structure
|
Common Name | N-Allylnormetazocine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 133005-41-1 | Molecular Weight | 293.83200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H24ClNO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of N-Allylnormetazocine hydrochloride(+)-N-Allylnormetazocine ((+)-SKF 10047) hydrochloride is a benzomorphan opioid with psychotomi metic effects. (+)-N-Allylnormetazocine hydrochloride is an opioid receptor antagonist with Ki values of 300 nM and 27 μM for σ1 and σ2 opioid receptors, respectively. (+)-N-Allylnormetazocine hydrochloride can be used for the research of neurological disease[1][2]. |
| Name | (+)-N-Allyl Normetazocine Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | (+)-N-Allylnormetazocine ((+)-SKF 10047) hydrochloride is a benzomorphan opioid with psychotomi metic effects. (+)-N-Allylnormetazocine hydrochloride is an opioid receptor antagonist with Ki values of 300 nM and 27 μM for σ1 and σ2 opioid receptors, respectively. (+)-N-Allylnormetazocine hydrochloride can be used for the research of neurological disease[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | (+)-N-Allylnormetazocine hydrochloride (1 nM) 对 µ,δ 和 κ 阿片受体的抑制率分别为 28.5%,2.5% 和 31%[1]。 (+)-N-Allylnormetazocine hydrochloride 对 σ1 和 σ2 阿片受体的 Ki 值分别为 300 nM 和 27 μM[1]。 |
| In Vivo | (+)-N-Allylnormetazocine hydrochloride (0.3,1,3,10 和 30 mg/kg;腹腔注射,每次实验前10 分钟) 剂量依赖性地增加解离性麻醉药苯环利定 (PCP) 的反应[2]。 |
| References |
| Molecular Formula | C17H24ClNO |
|---|---|
| Molecular Weight | 293.83200 |
| Exact Mass | 293.15500 |
| PSA | 23.47000 |
| LogP | 3.84230 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Allosteric modulation of sigma-1 receptors elicits anti-seizure activities.
Br. J. Pharmacol. 172 , 4052-65, (2015) Application of orthosteric sigma-1 receptor agonists as anti-seizure drugs has been hindered by questionable efficacy and potential adverse effects. Here, we have investigated the anti-seizure effects... |
|
|
Sigma1 recognition sites in rabbit iris-ciliary body: topical sigma1-site agonists lower intraocular pressure.
J. Pharmacol. Exp. Ther. 289 , 1362, (1999) In this study, we examined the presence of sigma1 and sigma2 sites in the rabbit iris-ciliary body by receptor binding and investigated their effects on intraocular pressure (IOP) in albino rabbits. T... |
|
|
sigma receptor ligands attenuate N-methyl-D-aspartate cytotoxicity in dopaminergic neurons of mesencephalic slice cultures.
Eur. J. Pharmacol. 388 , 139-146, (2000) We investigated the potential neuroprotective effects of several sigma receptor ligands in organotypic midbrain slice cultures as an excitotoxicity model system. When challenged with 100-microM N-meth... |
| (+)-N-Allylnormetazocine hydrochloride |