Rifampicin
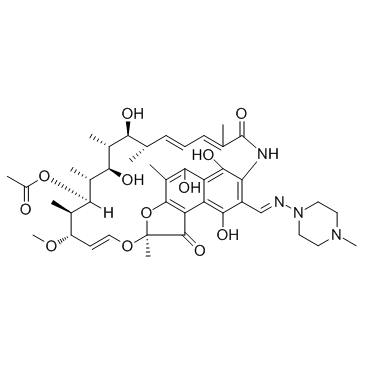
Rifampicin structure
|
Common Name | Rifampicin | ||
|---|---|---|---|---|
| CAS Number | 13292-46-1 | Molecular Weight | 822.940 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 1004.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C43H58N4O12 | Melting Point | 183ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 561.3±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of RifampicinRifampicin is a potent and broad spectrum antibiotic against bacterial pathogens. |
| Name | rifampicin |
|---|---|
| Synonym | More Synonyms |
| Description | Rifampicin is a potent and broad spectrum antibiotic against bacterial pathogens. |
|---|---|
| Related Catalog | |
| In Vitro | Rifampicin (100 mg/mL) can block the functional activity of P-glycoprotein. Rifampicin is not a substract for P-glycoprotein. The mechanism of rifampicin resistance is unassociated with the functional activity of P-glycoprotein[3]. |
| In Vivo | Rifampicin (200, 400 mg/kg) can induce fatty liver at high concentration[1]. Rifampicin (30 mg/kg, i.p.) treatment of S464P biofilms in vivo results in a slight decline, but earlier rebinds in bioluminescence from these catheters compared with the parental signal, whereas rifampicin has no affect on bioluminescence in mice infected with mutant H481Y[2]. |
| Animal Admin | Briefly, 1 cm Teflon catheter (14-gauge) carrying 104 cfu S. aureus, either the parental strain Xen 29 or the RifR mutants S464P or H481Y, are implanted subcutaneously in groups of nine mice per strain. One catheter segment is inserted on each side of each animal. Six days after the implantation of the catheters, five mice from each group are treated with rifampicin at 30 mg/kg intraperitoneally in 0.1 mL saline, twice daily for four consecutive days. The remaining four mice in each group are left untreated as controls. At various time points during the infection, the mice are anaesthetized using a constant flow of 1.5% isoflurane from the IVIS® manifold, and imaged using an IVIS® Image System 100 Series. The bioluminescent signals (photons/s) emitted from the mice are analysed using LivingImage® software and plotted over the course of infection. The mice are sacrificed 20 days after infection (11 days after final rifampicin treatment). The catheters are surgically removed and the bacteria are detached by sonication for determination of bacterial burdens on the catheters. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1004.4±65.0 °C at 760 mmHg |
| Melting Point | 183ºC (dec.) |
| Molecular Formula | C43H58N4O12 |
| Molecular Weight | 822.940 |
| Flash Point | 561.3±34.3 °C |
| Exact Mass | 822.405151 |
| PSA | 220.15000 |
| LogP | 1.09 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.613 |
| Storage condition | 2-8°C |
| Water Solubility | chloroform: soluble50mg/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VJ7000000 |
| HS Code | 2941903000 |
|
~% 
Rifampicin CAS#:13292-46-1 |
| Literature: US4174320 A1, ; |
|
~% 
Rifampicin CAS#:13292-46-1 |
| Literature: US4174320 A1, ; |
|
~% 
Rifampicin CAS#:13292-46-1 |
| Literature: US4174320 A1, ; |
|
~% 
Rifampicin CAS#:13292-46-1 |
| Literature: US4174320 A1, ; |
|
~% 
Rifampicin CAS#:13292-46-1 |
| Literature: US3963705 A1, ; |
| HS Code | 2941903000 |
|---|
|
Enhancement of in vitro activity of tuberculosis drugs by addition of thioridazine is not reflected by improved in vivo therapeutic efficacy.
Tuberculosis (Edinb.) 94(6) , 701-7, (2015) Assessment of the activity of thioridazine towards Mycobacterium tuberculosis (Mtb), in vitro and in vivo as a single drug and in combination with tuberculosis (TB) drugs.The in vitro activity of thio... |
|
|
Disruption of an M. tuberculosis membrane protein causes a magnesium-dependent cell division defect and failure to persist in mice.
PLoS Pathog. 11(2) , e1004645, (2015) The identification of Mycobacterium tuberculosis genes necessary for persistence in vivo provides insight into bacterial biology as well as host defense strategies. We show that disruption of M. tuber... |
|
|
Kinetics of recA and recX induction in drug-susceptible and MDR clinical strains of Mycobacterium tuberculosis.
J. Antimicrob. Chemother. 69(12) , 3199-202, (2014) To investigate and compare the expression of recA and recX, components of the SOS pathway, following rifampicin treatment in drug-susceptible and MDR clinical strains of Mycobacterium tuberculosis.Str... |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,27,29-Pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methyl-1-piperazinyl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyc ;lo[23.3.1.1.0]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-13-yl acetate |
| rifobac |
| Rimactane |
| arficin |
| 2,7-(Epoxy[1,11,13]pentadecatrienoimino)naphtho[2,1-b]furan-1,11(2H)-dione, 21-(acetyloxy)-5,6,9,17,19-pentahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-8-[(E)-[(4-methyl-1-piperazinyl)imino]methyl]-, (2S,12Z,14E,16S,17S,18R,19R,20R,21S,22R,23S,24E)- |
| Rifampicin |
| FaMcin |
| rifinah |
| Eremfat |
| Rimactan |
| rifagen |
| MFCD00151389 |
| Rifamycin AMP |
| 3-[[(4-Methyl-1-piperazinyl)imino]methyl]rifamycin |
| Rifadine |
| RIF |
| 3-(4-Methylpiperazinyliminomethyl)rifamycin SV,Rifampin,Rifamycin AMP |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,27,29-Pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methyl-1-piperazinyl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-13-yl acetate |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E)-2,15,17,27,29-Pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methylpiperazin-1-yl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-13-yl acetate |
| Rifa |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,27,29-pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methylpiperazin-1-yl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),1(29),2,4,9,19,21,25,27-nonaen-13-yl acetate |
| UNII-VJT6J7R4TR |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,27,29-Pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methylpiperazin-1-yl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-13-yl acetate |
| 2,7-(epoxy[1,11,13]pentadecatrienoimino)naphtho[2,1-b]furan-1,11(2H)-dione, 21-(acetyloxy)-5,6,9,17,19-pentahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-8-[(E)-[(4-methyl-1-piperazinyl)imino]methyl]-, (2S,14E,16S,17S,18R,19R,20R,21S,22R,23S,24E)- |
| EINECS 236-312-0 |
| (7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,27,29-Pentahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-26-{(E)-[(4-methylpiperazin-1-yl)imino]methyl}-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.1.0]triaconta-1(28),1(29),2,4,9,19,21,25,27-nonaen-13-ylacetat |
| (2S,12Z,14E,16S,17S,18R,19R,20R,21S,22R,23S,24E)-5,6,9,17,19-pentahydroxy-23-methoxy-2,4,12,16,18,20,22-heptamethyl-8-{(E)-[(4-methylpiperazin-1-yl)imino]methyl}-1,11-dioxo-1,2-dihydro-2,7-(epoxypentadeca[1,11,13]trienoimino)naphtho[2,1-b]furan-21-yl acetate |
| Abrifam |
| Rifampin |
| RIFADIN |
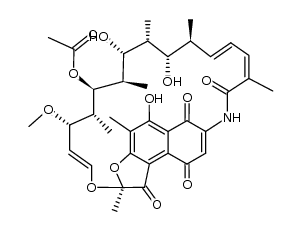

![4-[2-[3,5-bis(2-morpholin-4-ylethyl)-1,3,5-triazinan-1-yl]ethyl]morpholine structure](https://image.chemsrc.com/caspic/410/70935-97-6.png)

 CAS#:13292-22-3
CAS#:13292-22-3 CAS#:63624-40-8
CAS#:63624-40-8
