Angiotensin II Receptor Ligand
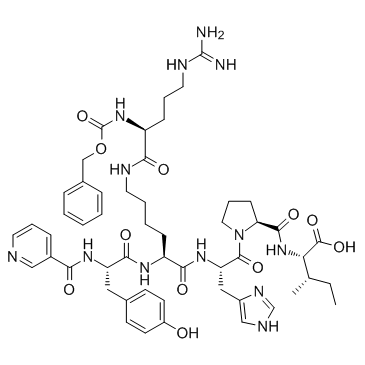
Angiotensin II Receptor Ligand structure
|
Common Name | Angiotensin II Receptor Ligand | ||
|---|---|---|---|---|
| CAS Number | 127060-75-7 | Molecular Weight | 1052.185 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C52H69N13O11 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Angiotensin II Receptor LigandCGP-42112(CGP-42112A) is a potent Angiotensin-II subtype 2 receptor(AT2 R) agonist.IC50 value:Target: AT2 R agonistin vitro: CGP42112 (>==1 nM) significantly inhibited cGMP production from the basal value. CGP42112 (>==1 nM) significantly inhibited TH-enzyme activity from the basal value. These inhibitory effects of CGP42112 on TH-enzyme activity and-cGMP production were abolished by PD123319 (AT(2)-R antagonist) while CV-11974 (AT(1)-R antagonist) was ineffective [1]. [125I]CGP 42112 bound selectively to the AT2 angiotensin II receptor subtype. [125I]CGP 42112 bound with higher affinity in the brain than in the adrenal. beta-Mercaptoethanol enhanced [125I]CGP 42112 binding in the brain, but did not alter its binding in the adrenal [2]. [125I]CGP 42112 bound with high affinity (Kd = 0.07-0.3 nM, depending on the area studied). [125I]CGP 42112 binding was selective for AT2 receptors, as determined by lack of competition with the AT1 ligand losartan, and competition by the AT2 ligands PD 123177 and unlabeled CGP 42112 and the non-selective peptides Ang II and angiotensin III (Ang III) [4].in vivo: Intravenous infusions of CGP 42112 (0.1 and 1 mg kg-1 min-1) and PD 123319 (0.36 and 1 mg kg-1 min-1) shifted the upper limit of CBF autoregulation toward higher blood pressures without affecting baseline CBF [3]. |
| Name | cgp 42112 |
|---|---|
| Synonym | More Synonyms |
| Description | CGP-42112(CGP-42112A) is a potent Angiotensin-II subtype 2 receptor(AT2 R) agonist.IC50 value:Target: AT2 R agonistin vitro: CGP42112 (>==1 nM) significantly inhibited cGMP production from the basal value. CGP42112 (>==1 nM) significantly inhibited TH-enzyme activity from the basal value. These inhibitory effects of CGP42112 on TH-enzyme activity and-cGMP production were abolished by PD123319 (AT(2)-R antagonist) while CV-11974 (AT(1)-R antagonist) was ineffective [1]. [125I]CGP 42112 bound selectively to the AT2 angiotensin II receptor subtype. [125I]CGP 42112 bound with higher affinity in the brain than in the adrenal. beta-Mercaptoethanol enhanced [125I]CGP 42112 binding in the brain, but did not alter its binding in the adrenal [2]. [125I]CGP 42112 bound with high affinity (Kd = 0.07-0.3 nM, depending on the area studied). [125I]CGP 42112 binding was selective for AT2 receptors, as determined by lack of competition with the AT1 ligand losartan, and competition by the AT2 ligands PD 123177 and unlabeled CGP 42112 and the non-selective peptides Ang II and angiotensin III (Ang III) [4].in vivo: Intravenous infusions of CGP 42112 (0.1 and 1 mg kg-1 min-1) and PD 123319 (0.36 and 1 mg kg-1 min-1) shifted the upper limit of CBF autoregulation toward higher blood pressures without affecting baseline CBF [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C52H69N13O11 |
| Molecular Weight | 1052.185 |
| Exact Mass | 1051.523926 |
| PSA | 365.14000 |
| LogP | 2.38 |
| Index of Refraction | 1.659 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
|
Altered efficacy of AT1R-targeted treatment after spontaneous cancer cell-AT1R upregulation.
BMC Cancer 11 , 274, (2011) Targeting of the renin angiotensin system (RAS) reduces tumour growth in experimental models of cancer. We aimed to establish if combined targeting of the 'classical' and 'alternative' arms of the RAS... |
|
|
Update on the angiotensin AT(2) receptor.
Curr. Hypertens. Rep. 15(1) , 25-30, (2013) It is quite well established that activation of the AT(2) receptor (AT(2)R) provides a counter-regulatory role to AT(1)R overactivity, particularly during pathological conditions. Indeed, a potential ... |
|
|
Angiotensin III modulates the nociceptive control mediated by the periaqueductal gray matter
Neuroscience 164(3) , 1263-73, (2009) Endogenous angiotensin (Ang) II and/or an Ang II-derived peptide, acting on Ang type 1 (AT1) and Ang type 2 (AT2) receptors, can carry out part of the nociceptive control modulated by periaqueductal g... |
| L-Isoleucine, N-[N-(diaminomethylene)-N-[(phenylmethoxy)carbonyl]-L-ornithyl]-N-[N-(3-pyridinylcarbonyl)-L-tyrosyl]-L-lysyl-L-histidyl-L-prolyl- |
| MFCD00133611 |
| N-{N-[(Benzyloxy)carbonyl]-N-(diaminomethylene)-L-ornithyl}-N-[N-(3-pyridinylcarbonyl)-L-tyrosyl]-L-lysyl-L-histidyl-L-prolyl-L-isoleucine |
| CGP-42112 |