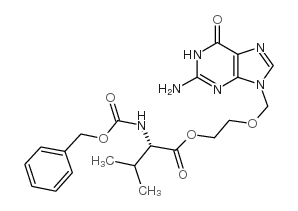
Valacyclovir hydrochloride
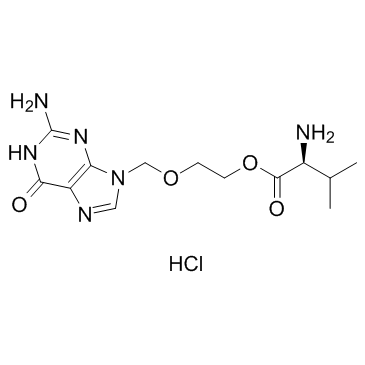
Valacyclovir hydrochloride structure
|
Common Name | Valacyclovir hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 124832-27-5 | Molecular Weight | 360.797 | |
| Density | 1.55g/cm3 | Boiling Point | 588.4ºC at 760 mmHg | |
| Molecular Formula | C13H21ClN6O4 | Melting Point | 170-172ºC | |
| MSDS | Chinese USA | Flash Point | 309.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Valacyclovir hydrochlorideValacyclovir hydrochloride is an antiviral drug used in the management of herpes simplex, herpes zoster, and herpes B. Target: HSVValacyclovir is an antiviral drug used in the management of herpes simplex, herpes zoster, and herpes B. VACV uptake was concentration dependent and saturable with a Michaelis-Menten constant and maximum velocity of 1.64 +/- 0.06 mM and 23.34 +/- 0.36 nmol/mg protein/5 min, respectively. A very similar Km value was obtained in hPEPT1/CHO cells and in rat and rabbit tissues and Caco-2 cells, suggesting that hPEPT1 dominates the intestinal transport properties of VACV in vitro . For treatment of a first episode of genital herpes, a large comparative trial has shown that valacyclovir (1 g twice a day) is as effective as acyclovir (200 mg five times a day) when given for 10 days. For treating recurrences, two trials show that valacyclovir is as effective as acyclovir (200 mg five times a day) with a treatment period of 5 days. A daily dose of 1 g of valacyclovir is as effective as 2 g daily. Valacyclovir can be administered once a day. The concentrations of acyclovir in serum and CSF were measured at steady state after 6 days of oral treatment with 1,000 mg of valacyclovir three times a day. EC50 values of PE and AC in 3T3 cells were 0.02 and 0.01 ug/ml, while values in BHK cells were 0.2 and 0.03 ug/ml. Treatment of infected immunosuppressed mice and FA and VA (b.i.d., 5.5 days) reduced the proportion with erythema from 100% to 24% and 38%, and eliminated ear paralysis, ear lesions (vesicles, etc) and death. Virus was absent from ear and brainstem by day 6, but reappeared after discontinuation in mice treated with VA. |
| Name | Valacyclovir Hydrochloride Hydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Valacyclovir hydrochloride is an antiviral drug used in the management of herpes simplex, herpes zoster, and herpes B. Target: HSVValacyclovir is an antiviral drug used in the management of herpes simplex, herpes zoster, and herpes B. VACV uptake was concentration dependent and saturable with a Michaelis-Menten constant and maximum velocity of 1.64 +/- 0.06 mM and 23.34 +/- 0.36 nmol/mg protein/5 min, respectively. A very similar Km value was obtained in hPEPT1/CHO cells and in rat and rabbit tissues and Caco-2 cells, suggesting that hPEPT1 dominates the intestinal transport properties of VACV in vitro . For treatment of a first episode of genital herpes, a large comparative trial has shown that valacyclovir (1 g twice a day) is as effective as acyclovir (200 mg five times a day) when given for 10 days. For treating recurrences, two trials show that valacyclovir is as effective as acyclovir (200 mg five times a day) with a treatment period of 5 days. A daily dose of 1 g of valacyclovir is as effective as 2 g daily. Valacyclovir can be administered once a day. The concentrations of acyclovir in serum and CSF were measured at steady state after 6 days of oral treatment with 1,000 mg of valacyclovir three times a day. EC50 values of PE and AC in 3T3 cells were 0.02 and 0.01 ug/ml, while values in BHK cells were 0.2 and 0.03 ug/ml. Treatment of infected immunosuppressed mice and FA and VA (b.i.d., 5.5 days) reduced the proportion with erythema from 100% to 24% and 38%, and eliminated ear paralysis, ear lesions (vesicles, etc) and death. Virus was absent from ear and brainstem by day 6, but reappeared after discontinuation in mice treated with VA. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.55g/cm3 |
|---|---|
| Boiling Point | 588.4ºC at 760 mmHg |
| Melting Point | 170-172ºC |
| Molecular Formula | C13H21ClN6O4 |
| Molecular Weight | 360.797 |
| Flash Point | 309.7ºC |
| Exact Mass | 360.131287 |
| PSA | 151.14000 |
| LogP | 1.28590 |
| Vapour Pressure | 7.95E-14mmHg at 25°C |
| Index of Refraction | 1.673 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~99% 
Valacyclovir hy... CAS#:124832-27-5 |
| Literature: Prasada Raju; Vedantham, Ravindra; Khunt, Mayur D.; Mathad, Vijayavitthal T.; Dubey, Pramod K.; Chakravarthy, Akula Kalyan Asian Journal of Chemistry, 2010 , vol. 22, # 5 p. 4092 - 4098 |
|
~% 
Valacyclovir hy... CAS#:124832-27-5 |
| Literature: US2007/112193 A1, ; Page/Page column 8 ; |
|
~% 
Valacyclovir hy... CAS#:124832-27-5 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 90, # 10 p. 1505 - 1515 |
|
~% 
Valacyclovir hy... CAS#:124832-27-5 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 90, # 10 p. 1505 - 1515 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients.
Cochrane Database Syst. Rev. 8 , CD009036, (2014) Genital herpes is caused by herpes simplex virus 1 (HSV-1) or 2 (HSV-2). Some infected people experience outbreaks of genital herpes, typically, characterized by vesicular and erosive localized painfu... |
|
|
Acute retinal necrosis associated with Epstein-Barr virus: immunohistopathologic confirmation.
JAMA Ophthalmol. 132(7) , 881-2, (2014) Acute retinal necrosis (ARN) is an infectious retinitis primarily caused by the herpesviruses. Although the Epstein-Barr virus (EBV) has been implicated as a cause of ARN, to our knowledge, there has ... |
|
|
A pilot study examining the safety and tolerability of valacyclovir in veterans with hepatitis C virus/herpes simplex virus type 2 coinfection.
Am. J. Med. Sci. 348(6) , 455-9, (2014) We performed a pilot study examining the safety and tolerability of valacyclovir in veterans with herpes simplex virus type 2 and hepatitis C virus (HCV) coinfection.We performed a randomized double-b... |
| 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester monohydrochloride |
| MFCD01861507 |
| 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl L-valinate hydrochloride |
| L-Valine 2-(2-Amino-1,6-dihydro-6-oxo-9H-purin-9-ylmethoxy)ethyl Ester Hydrochloride Hydrate |
| Valaciclovir |
| 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl valinate hydrochloride (1:1) |
| 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl-(2S)-2-amino-3-methylbutanoathydrochlorid |
| 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate hydrochloride |
| Valacyclovir hydrochloride |
| Zelitrex |
| Valaciclovir hydrochloride |
| (2S)-2-amino-3-méthylbutanoate de 2-[(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)méthoxy]éthyle chlorhydrate |
| Valaciclovir HCl |
| 256u |
| L-Valine 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester monohydrochloride |
| Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]ethyl ester, hydrochloride (1:1) |
| Valacyclovir hydrochloride hydrate |
| Valacyclovir (hydrochloride) |