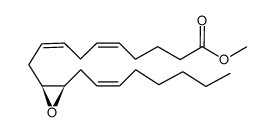
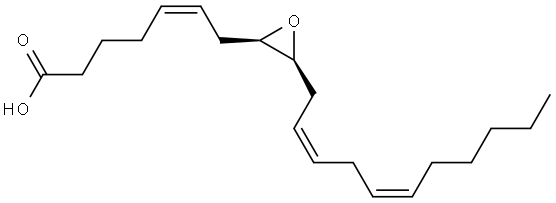
(11S,12R)-EET
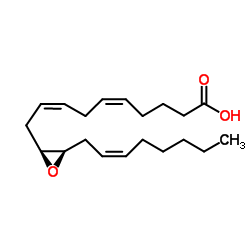
(11S,12R)-EET structure
|
Common Name | (11S,12R)-EET | ||
|---|---|---|---|---|
| CAS Number | 123931-40-8 | Molecular Weight | 320.466 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 461.8±33.0 °C at 760 mmHg | |
| Molecular Formula | C20H32O3 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 154.6±18.9 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of (11S,12R)-EET(±)11(12)-EET is a NLRP3 inflammasome inhibitor. (±)11(12)-EET can be used for the research of anti-inflammatory, angiogenic and cardioprotective[1][2][3][4][6]. |
| Name | 11,12-Epoxy-(5Z,8Z,14Z)-eicosatrienoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (±)11(12)-EET is a NLRP3 inflammasome inhibitor. (±)11(12)-EET can be used for the research of anti-inflammatory, angiogenic and cardioprotective[1][2][3][4][6]. |
|---|---|
| Related Catalog | |
| Target |
NLRP3 inflammasome |
| In Vitro | (±)11(12)-EET (5 μM; 10 mimutes; macrophages) depresses NLRP3 protein expression, dramatically decreases the expression of pro-IL-1β in cells and the supernatant and reduces the intracellular ROS[1]. Western Blot Analysis[1] Cell Line: Macrophages Concentration: 5 μM Incubation Time: 10 mimutes Result: Depresses NLRP3 protein expression. Immunofluorescence[1] Cell Line: Macrophages Concentration: 5 μM Incubation Time: 10 mimutes Result: Reduced the intracellular ROS. |
| In Vivo | (±)11(12)-EET increases adhesion of isolated peripheral blood leukocytes in a chamber coated with P-selectin and ICAM-1 in 50 µg/kg[5]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 461.8±33.0 °C at 760 mmHg |
| Molecular Formula | C20H32O3 |
| Molecular Weight | 320.466 |
| Flash Point | 154.6±18.9 °C |
| Exact Mass | 320.235138 |
| PSA | 49.83000 |
| LogP | 6.30 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.501 |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338-P337 + P313-P403 + P235 |
| RIDADR | UN1170 - class 3 - PG 2 - Ethanol, solution |
|
~% 
(11S,12R)-EET CAS#:123931-40-8 |
| Literature: Mosset, Paul; Yadagiri, Pendri; Lumin, Sun; Capdevila, Jorge; Falck, J. R. Tetrahedron Letters, 1986 , vol. 27, # 50 p. 6035 - 6038 |
|
~% 
(11S,12R)-EET CAS#:123931-40-8 |
| Literature: Mosset, Paul; Yadagiri, Pendri; Lumin, Sun; Capdevila, Jorge; Falck, J. R. Tetrahedron Letters, 1986 , vol. 27, # 50 p. 6035 - 6038 |
|
~% 
(11S,12R)-EET CAS#:123931-40-8 |
| Literature: Mosset, Paul; Yadagiri, Pendri; Lumin, Sun; Capdevila, Jorge; Falck, J. R. Tetrahedron Letters, 1986 , vol. 27, # 50 p. 6035 - 6038 |
|
~% 
(11S,12R)-EET CAS#:123931-40-8
Detail
|
| Literature: Mesaros, Clementina; Lee, Seon Hwa; Blair, Ian A. Rapid Communications in Mass Spectrometry, 2010 , vol. 24, # 22 p. 3237 - 3247 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Protective effects of n-6 fatty acids-enriched diet on intestinal ischaemia/reperfusion injury involve lipoxin A4 and its receptor.
Br. J. Pharmacol. 172(3) , 910-23, (2015) Long-term intake of dietary fatty acids is known to predispose to chronic inflammation, but their effects on acute intestinal ischaemia/reperfusion (I/R) injury is unknown. The aim of this study was t... |
|
|
Cyp2c44 epoxygenase in the collecting duct is essential for the high K+ intake-induced antihypertensive effect.
Am. J. Physiol. Renal Physiol. 307(4) , F453-60, (2014) Cytochrome P-450, family 2, subfamily c, polypeptide 44 (Cyp2c44) epoxygenase metabolizes arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs) in kidney and vascular tissues. In the present study... |
|
|
UHPLC-MS/MS analysis of arachidonic acid and 10 of its major cytochrome P450 metabolites as free acids in rat livers: effects of hepatic ischemia.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 964 , 153-63, (2014) The cytochrome P450 metabolites of arachidonic acid (AA) are mostly present in tissues, such as the liver, as bound to phospholipids, with only a small fraction available as free acids. The purpose of... |
| 5,8-Decadienoic acid, 10-[(2S,3R)-3-[(2Z)-2-octen-1-yl]oxiranyl]-, (5Z,8Z)- |
| (5Z,8Z)-10-{(2S,3R)-3-[(2Z)-2-Octen-1-yl]-2-oxiranyl}-5,8-decadienoic acid |
| (11S,12R)-EET |
| (5Z,8Z)-10-{(2S,3R)-3-[(2Z)-oct-2-en-1-yl]oxiran-2-yl}deca-5,8-dienoic acid |
| (5Z,8Z)-10-{(2S,3R)-3-[(2Z)-2-Octen-1-yl]-2-oxiranyl}-5,8-decadie noic acid |