MK-886 (sodium salt)
Modify Date: 2025-08-25 00:50:43
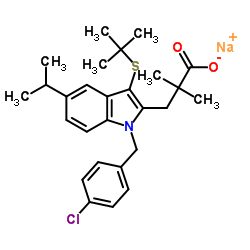
MK-886 (sodium salt) structure
|
Common Name | MK-886 (sodium salt) | ||
|---|---|---|---|---|
| CAS Number | 118427-55-7 | Molecular Weight | 494.06 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H33ClNNaO2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of MK-886 (sodium salt)MK-886 (L 663536) sodium salt is a potent, cell-permeable and orally active FLAP (IC50 of 30 nM) and leukotriene biosynthesis (IC50s of 3 nM and 1.1 μM in intact leukocytes and human whole blood, respectively) inhibitor. MK-886 sodium salt is also a non-competitive PPARα antagonist and can induce apoptosis[1][2][3]. |
| Name | sodium,3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-propan-2-ylindol-2-yl]-2,2-dimethylpropanoate |
|---|---|
| Synonym | More Synonyms |
| Description | MK-886 (L 663536) sodium salt is a potent, cell-permeable and orally active FLAP (IC50 of 30 nM) and leukotriene biosynthesis (IC50s of 3 nM and 1.1 μM in intact leukocytes and human whole blood, respectively) inhibitor. MK-886 sodium salt is also a non-competitive PPARα antagonist and can induce apoptosis[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 30 nM (FLAP)[3] IC50: 3 nM (Leukotriene biosynthesis in intact leukocytes) and 1.1 μM (Leukotriene biosynthesis in human whole blood)[2] PPARα[1] |
| In Vitro | MK-886 sodium salt (0.5-2 μM; 15?hours; primary keratinocytes) treatment reduces keratin-1 expression in a culture of mouse primary keratinocytes[1]. ?Using a transient transfection system in monkey kidney fibroblast CV-1 cells, mouse keratinocyte 308 cells and human lung adenocarcinoma A549 cells, 10 μM MK-886 sodium salt is able to inhibit Wy-14643 activation of PPARα by ~80%. MK-886 sodium salt also decreases PPARα activation by fatty acids in the stable transfection system[1]. ?Although Jurkat cells express all PPAR isoforms, various PPARα and PPARγ agonists are unable to prevent MK-886 sodium salt-induced apoptosis[1]. |
| In Vivo | MK-886 sodium salt (L 663536; 5 mg/kg; oral administration; male Sprague-Dawley rats) treatment potently inhibits the antigen-induced dyspnea in inbred rats pretreated with methysergide[2]. ?MK-886 sodium salt (L 663536) inhibits leukotriene biosynthesis in vivo in a rat pleurisy model (ED50, 0.2 mg/kg p.o.), an inflamed rat paw model (ED50, 0.8 mg/kg), a model of leukotriene excretion in rat bile following antigen provocation[2]. |
| References |
| Molecular Formula | C27H33ClNNaO2S |
|---|---|
| Molecular Weight | 494.06 |
| Exact Mass | 493.181824 |
| PSA | 70.36000 |
| LogP | 6.67560 |
| MK-886 sodium salt hydrate |
| Sodium 3-[3-(tert-butylsulfanyl)-1-(4-chlorobenzyl)-5-isopropyl-1H-indol-2-yl]-2,2-dimethylpropanoate |
| Lopac-M-2692 |
| 1H-Indole-2-propanoic acid, 1-[(4-chlorophenyl)methyl]-3-[(1,1-dimethylethyl)thio]-α,α-dimethyl-5-(1-methylethyl)-, sodium salt (1:1) |
| MK-886 sodium salt |
| MK-886 |
| Sodium 3-{1-(4-chlorobenzyl)-5-isopropyl-3-[(2-methyl-2-propanyl)sulfanyl]-1H-indol-2-yl}-2,2-dimethylpropanoate |
| MK-886 sodium hydrate |