N-Desmethyl levofloxacin
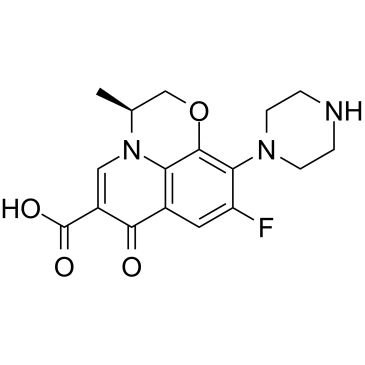
N-Desmethyl levofloxacin structure
|
Common Name | N-Desmethyl levofloxacin | ||
|---|---|---|---|---|
| CAS Number | 117707-40-1 | Molecular Weight | 347.34100 | |
| Density | 1.517g/cm3 | Boiling Point | 600.714ºC at 760 mmHg | |
| Molecular Formula | C17H18FN3O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 317.101ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of N-Desmethyl levofloxacinDesmethyl Levofloxacin is a metabolite of Levofloxacin. Levofloxacin, a synthetic fluoroquinolone, is an antibacterial agent that inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication[1]. |
| Name | S-(-)-9-fluoro-2,3-dihydro-3-methyl-10-(1-piperazinyl)-7-oxo-7H-pyrido-<1,2,3-de><1,4>benzoxazine-6-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Desmethyl Levofloxacin is a metabolite of Levofloxacin. Levofloxacin, a synthetic fluoroquinolone, is an antibacterial agent that inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.517g/cm3 |
|---|---|
| Boiling Point | 600.714ºC at 760 mmHg |
| Molecular Formula | C17H18FN3O4 |
| Molecular Weight | 347.34100 |
| Flash Point | 317.101ºC |
| Exact Mass | 347.12800 |
| PSA | 83.80000 |
| LogP | 1.59560 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.677 |
| InChIKey | WKRSSAPQZDHYRV-VIFPVBQESA-N |
| SMILES | CC1COc2c(N3CCNCC3)c(F)cc3c(=O)c(C(=O)O)cn1c23 |
|
~78% 
N-Desmethyl lev... CAS#:117707-40-1 |
| Literature: UNIVERSITY OF MARYLAND, BALTIMORE; MITSUBISHI TANABE PHARMA CORPORATION Patent: WO2009/64836 A2, 2009 ; Location in patent: Page/Page column 13-14 ; WO 2009/064836 A2 |
|
~81% 
N-Desmethyl lev... CAS#:117707-40-1 |
| Literature: Korea Institute of Science and Technology Patent: US5539110 A1, 1996 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Pharmacokinetics and pharmacodynamics of fluoroquinolones. Rodvold, Keith A., and Melinda Neuhauser.
Pharmacotherapy 21.10P2 , 233S-252S, (2001)
|
| UNII-88ZBA45NC8 |
| Levofloxacin related compound A |
| Uing 4-255 |
| des-methyl levofloxacin |
| N-Desmethyl levofloxacin |
| Levofloxacin Impurity 2 |