CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UU8815550
-
CHEMICAL NAME :
-
7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid, 2,3-dihydro-9-fluoro-3-methyl- 10-(4-methyl-1-piperazinyl)-7-oxo-, hemihydrate, (S)-
-
CAS REGISTRY NUMBER :
-
100986-85-4
-
LAST UPDATED :
-
199403
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C18-H20-F-N3-O4.1/2H2-O
-
MOLECULAR WEIGHT :
-
342.41
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1478 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,365,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1803 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,365,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
>250 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,365,1992 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
14560 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Kidney, Ureter, Bladder - other changes in urine composition Blood - changes in leukocyte (WBC) count
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,367,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
22400 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - changes in phosphorus
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 16,29,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
2800 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume decreased Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 16,29,1991 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8910 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,374,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8910 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 42,374,1992
|
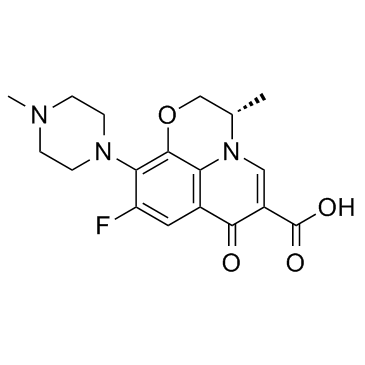




 CAS#:109-01-3
CAS#:109-01-3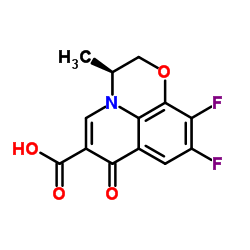 CAS#:100986-89-8
CAS#:100986-89-8 CAS#:50398-09-9
CAS#:50398-09-9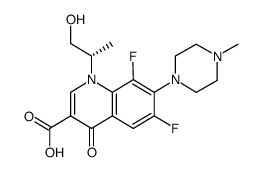 CAS#:1036016-10-0
CAS#:1036016-10-0 CAS#:177472-29-6
CAS#:177472-29-6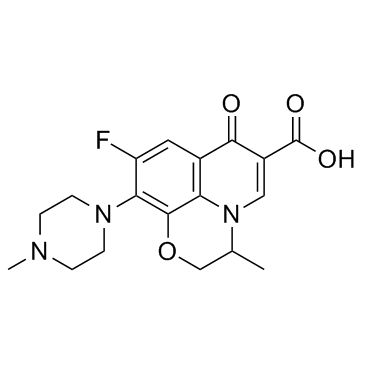 CAS#:82419-36-1
CAS#:82419-36-1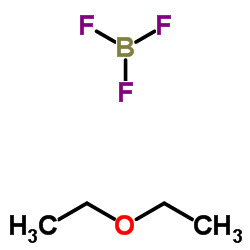 CAS#:109-63-7
CAS#:109-63-7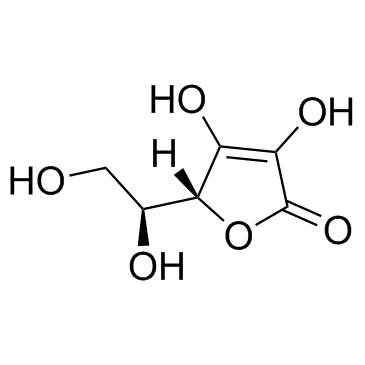 CAS#:50-81-7
CAS#:50-81-7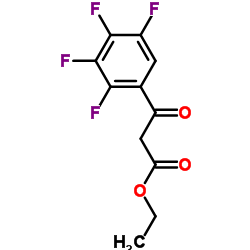 CAS#:94695-50-8
CAS#:94695-50-8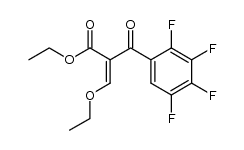 CAS#:103995-33-1
CAS#:103995-33-1
