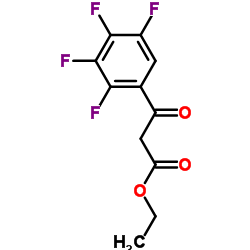
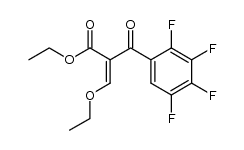
100986-85-4
| Name | levofloxacin |
|---|---|
| Synonyms |
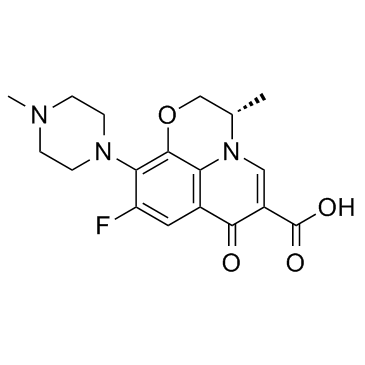
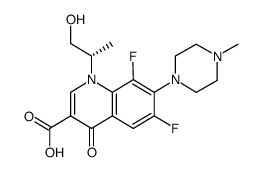
(3S)-9-Fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid
Levofloxacin L-Ofloxacin S-(-)-Ofloxacin MFCD03265511 Levaquin levoflaxacin (3S)-9-Fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Tavanic |
| Description | Levofloxacin, a synthetic fluoroquinolone, is an antibacterial agent that inhibits the supercoiling activity of bacterial DNA gyrase, halting DNA replication.Target: AntibacterialLevofloxacin reduced bacterial load compared with placebo by 4.9-fold (95% confidence interval, 1.4-25.7; P=0.02) at day 7 but had no effect at any point on any marker of neutrophilic airway inflammation. In patients with a baseline bacterial load of more than 10(6) cfu/mL, levofloxacin treatment was associated with a 26.5% (95% confidence interval, 1.8%-51.3%; P=0.04) greater reduction in the percentage neutrophil count compared with placebo at day 7 [1]. Levofloxacin was found to significantly improve the clinical and microbiological parameters in CP individuals [2]. A 30-day course of levofloxacin does not significantly improve BK viral load reduction or allograft function when used in addition to overall reduction of immunosuppression [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 571.5±50.0 °C at 760 mmHg |
| Melting Point | 218ºC |
| Molecular Formula | C18H20FN3O4 |
| Molecular Weight | 361.367 |
| Flash Point | 299.4±30.1 °C |
| Exact Mass | 361.143799 |
| PSA | 75.01000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.670 |
| Storage condition | Store at 0-5°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H317-H334-H361d-H362 |
| Precautionary Statements | P261-P263-P280-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26-36/37/39-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UU8815550 |
| HS Code | 2934999090 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |