1211866-85-1
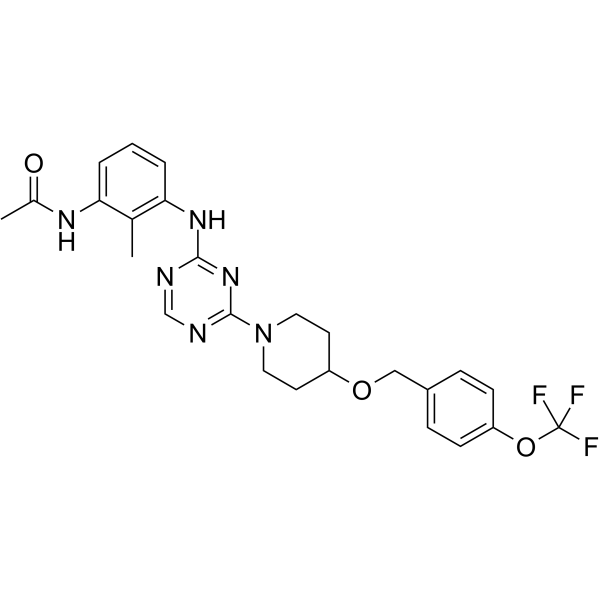
| Name | N-(2-Methyl-3-{[4-(4-{[4-(trifluoromethoxy)benzyl]oxy}-1-piperidi nyl)-1,3,5-triazin-2-yl]amino}phenyl)acetamide |
|---|---|
| Synonyms |
Cyclohexanamine,N-(2-methyl-2-propenylidene)-,(E)
N-(2-methyl-3-(4-(4-(4-(trifluoromethoxy)benzyloxy)piperidin-1-yl)-1,3,5-triazin-2-ylamino)phenyl)acetamide N-(2-methyl-2-propen-1-ylidene)cyclohexylamine Cyclohexanamine,N-(2-methyl-2-propenylidene) |
| Description | TC-N 1752 is a potent and orally active inhibitor of Nav1.7, with IC50s of 0.17 μM, 0.3 μM, 0.4 μM, 1.1 μM and 2.2 μM at hNav1.7, hNav1.3, hNav1.4, hNaV1.5 and rNav1.8, respectively. TC-N 1752 also inhibits tetrodotoxin-sensitive sodium channels. TC-N 1752 shows analgesic efficacy in the Formalin model of pain[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
hNav1.7:0.17 μM (IC50) hNav1.3:0.3 μM (IC50) hNav1.4:0.4 μM (IC50) hNav1.5:1.1 μM (IC50) hNav1.8:0.1 μM (IC50) |
| In Vitro | TC-N 1752 (compound 52) state-dependently inhibits Nav1.7, with IC50 of 170 nM on channels that are 20% inactivated and IC50 of 3.6 μM on fully noninactivated channels[1]. TC-N 1752 inhibits hNav1.7, hNav1.8, hNav1.9, rNav1.9, and mNav1.9 with IC50s of 0.2, 0.1, 1.6, 0.5 and 1.4 μM, respectively[2]. |
| In Vivo | TC-N 1752 (compound 52) (3-30 mg/kg; p.o.) dose-dependently shows analgesic effect in the Formalin model[1]. TC-N 1752 (3-30 mg/kg; p.o.) decreases thermal hyperalgesia produced by inflammation[3]. TC-N 1752 (5 mg/mL; 500 μL; i.v.) attenuates complete Freund’s adjuvant (CFA)-induced sensitization of C-fiber nociceptors[3]. Animal Model: Rats were injected intraplantar with Formalin[1] Dosage: 3, 10, 20, 30 mg/kg Administration: Administered p.o. 120 min prior to Formalin Result: Showed analgesic efficacy starting at the dose of 3 mg/kg, with full efficacy at 20 mg/kg dose. |
| References |
| Density | 1.37±0.1 g/cm3(Predicted) |
|---|---|
| Molecular Formula | C25H27F3N6O3 |
| Molecular Weight | 516.52 |
| Exact Mass | 516.21000 |
| PSA | 108.22000 |
| LogP | 5.10260 |
| Storage condition | 2-8°C |
| Precursor 6 | |
|---|---|
| DownStream 0 | |