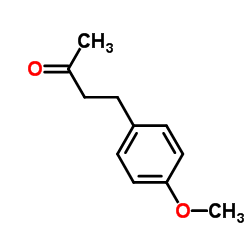
5471-51-2
| Name | raspberry ketone |
|---|---|
| Synonyms |
Oxyphenalon
4-hydroxy benzyl acetone Raspberry keton 4-(4-Hydroxyphenyl)-2-butanone Frambinone Rheosmin OXYPHENONE OXYPHENYLON Raspberry ketone EINECS 226-806-4 RASBERRY KETONE MFCD00002394 p-Hydroxy benzylacetone FEMA 2588 OXANONE |
| Description | Raspberry ketone is a major aromatic compound of red raspberry, widely used as a fragrance in cosmetics and as a flavoring agent in foodstuff; also shows PPAR-α agonistic activity. |
|---|---|
| Related Catalog | |
| Target |
PPAR-α |
| In Vitro | Raspberry ketone (1, 10, 20, and 50 μM) suppresses adipogenesis and lipid accumulation in 3T3-L1 pre-adipocytes. Raspberry ketone (10 µM) significantly blocks C/EBPα, PPARγ, and aP2 expression and increases the expression of ATGL and HSL, and CPT1B[1]. |
| In Vivo | Raspberry ketone (0.5%, 1%, or 2%) increasses the levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol contents (LDL-C), ISI (insulin-sensitivr index), PPAR-α and LDLR, decreases the serum levels of AST (aspartate aminotransferase), ALT (alanine aminotransferase), ALP (alkaline phosphatase), IRI (insulin resistance index), GLU (glucose), INS (insulin-sensitivr index), LEP (leptin), and TNF-α in rats compared with a high-fat diet-induced NASH model. Raspberry ketone also causes increased SOD activities[2]. Raspberry ketone shows cardioprotective action against isoproterenol-induced myocardial infarction in rats, and the effects may be due to its PPAR-α agonistic activity[3]. |
| Cell Assay | For the cytotoxicity study, 3T3-L1 pre-adipocytes are cultured and differentiated. After Raspberry ketone treatment for 4 d in DMEM containing 10% fetal bovine serum, the lactate dehydrogenase (LDH) concentration in the medium is immediately detected with the CytoTox 96 nonradioactive cytotoxicity assay kit[1]. |
| Animal Admin | During the experimental period, the animal room holds four rats per cage, with free access to water and food, under conditions of temperature controlled at 20-26°C, humidity at 40-70%, and a 12/12-h day-night light cycle. Rats are fed with normal diet for 1 week and then randomly divided into five groups: normal control (NC) group (n=8) fed normal diet for 8 weeks, the model control (MC) group (n=8) fed high-fat diet (82% standard diet, 8.3% yolk powder, 9.0% lard, 0.5% cholesterol, and 0.2% sodium taurocholate), the Raspberry ketone low-dose (RKL) group (n=8), the Raspberry ketone middle-dose (RKM) group (n=8), and the Raspberry ketone high-dose (RKH) group (n=8). Rats are first fed with high-fat diet for 4 weeks, and then these rats are given intragastrically 0.5%, 1%, or 2% Raspberry ketone. The first two groups of rats are intragastrically administered salad oil at the same dose (2 mL/day per rat) once a day at 10:00 a.m., lasting for 4 weeks[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 292.2±15.0 °C at 760 mmHg |
| Melting Point | 81-85 °C(lit.) |
| Molecular Formula | C10H12O2 |
| Molecular Weight | 164.201 |
| Flash Point | 122.9±13.0 °C |
| Exact Mass | 164.083725 |
| PSA | 37.30000 |
| LogP | 0.94 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | EL8925000 |
| HS Code | 2914501100 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914501100 |
|---|---|
| Summary | HS: 2914501100. 4-(4-hydroxyphenyl)butan-2-one. VAT:17.0%. tax rebate rate:9.0%. supervision conditions:None. MFN tarrif:5.5%. general tariff:30.0% |


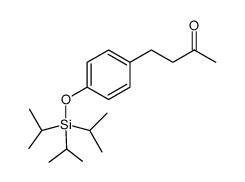

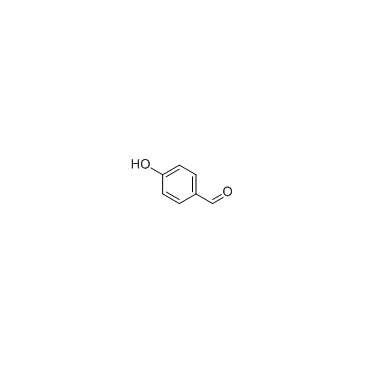

![2-[4-(3-hydroxy-3-methylbutyl)phenoxy]acetic acid structure](https://image.chemsrc.com/caspic/497/101268-32-0.png)