95635-56-6
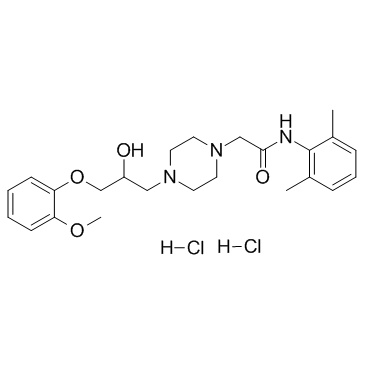
| Name | Ranolazine dihydrochloride |
|---|---|
| Synonyms |
Ranolazine dihydrochloride
RANOLAZINE HCL MFCD03788770 RANOLAZINE DI HCL Ranolazine (dihydrochloride) N-(2,6-Dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-1-piperazinyl}acetamide dihydrochloride Ranolazine DiHCI Ranolazine and salt 1-Piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-, hydrochloride (1:2) Ranolazine Dihydrocloride Ranolazine 2HCl N-(2,6-Dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}acetamide dihydrochloride Ranolazine dihydroch |
| Description | Ranolazine(RS-43285) is an antianginal agent with antiarrhythmic properties that achieves its effects via a novel mechanism of action (inhibition of the late phase of the inward sodium current), without affecting heart rate or blood pressure (BP). IC50 value:Target: sodium-dependent calcium channelRanolazine is currently approved for use in chronic angina. The basis for this use is likely related to inhibition of late sodium channels with resultant beneficial downstream effects. Randomized clinical trials have demonstrated an improvement in exercise capacity and reduction in angina episodes with ranolazine. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 624.1ºC at 760 mmHg |
|---|---|
| Melting Point | 222-229.5ºC(lit.) |
| Molecular Formula | C24H35Cl2N3O4 |
| Molecular Weight | 500.458 |
| Exact Mass | 499.200470 |
| PSA | 74.27000 |
| LogP | 3.86080 |
| Appearance | solid | off-white |
| Storage condition | Desiccate at RT |
| Water Solubility | H2O: 10 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2942000000 |
| HS Code | 2942000000 |
|---|