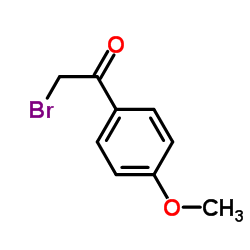
82640-04-8
| Name | raloxifene hydrochloride |
|---|---|
| Synonyms |
[6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl][4-(2-piperidin-1-ylethoxy)phenyl]methanone hydrochloride
[6-Hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]{4-[2-(piperidin-1-yl)ethoxy]phenyl}methanone hydrochloride (1:1) Raloxifene HCl [6-Hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl][4-(2-piperidin-1-ylethoxy)phenyl]methanonhydrochlorid 1-[2-(4-{[6-Hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]carbonyl}phenoxy)ethyl]piperidinium chloride [6-Hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]{4-[2-(1-piperidinyl)ethoxy]phenyl}methanone hydrochloride (1:1) Methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-, hydrochloride (1:1) methanone, [6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]-, hydrochloride 6-Hydroxy-2-(p-hydroxyphenyl)benzo(b)thien-3-yl-p-(2-piperidinoethoxy)phenyl ketone hydrochloride MFCD01938233 Raloxifene hydrochloride Raloxifene (hydrochloride) |
| Description | Raloxifene hydrochloride(LY156758 hydrochloride) is a second generation selective estrogen receptor antagonist.Target: Estrogen receptorApproved: September 14, 2007Raloxifene activates TGF beta 3 promoter as a full agonist at nanomolar concentrations, and raloxifene inhibits the estrogen response element-containing vitellogenin promoter expression as a pure estrogen antagonist in transient transfection assays [1]. Raloxifene, has been demonstrated as a potent uncompetitive inhibitor of human liver aldehyde oxidase-catalyzed oxidation of phthalazine, vanillin, and nicotine-Delta1'(5')-iminium ion, with Ki values of 0.87 nM, 1.2 nM and 1.4 nM. Raloxifene has also been shown to be a noncompetitive inhibitor of an aldehyde oxidase-catalyzed reduction reaction of a hydroxamic acid-containing compound, with a Ki of 51 nM [2]. Raloxifene (3 mg/kg/day) has potent estrogenic activity on bone resorption and serum cholesterol, a lesser effect on bone formation, and minimal activity on uterine wet weight in ovariectomized (OVX) rats. [3]. Raloxifene (0.1 mg/kg-10 mg/kg, orally for 5 weeks) increases bone mineral density in the distal femur and proximal tibia in ovariectomized (OVX) rat. Raloxifene reduces serum cholesteroloral with ED50 of 0.2 mg/kg in ovariectomized (OVX) rat. Raloxifene diverges dramatically from estrogen in its lack of significant estrogenic effects on uterine tissue [4]. Raloxifene prevents cancellous osteopenia as well as the changes in radial bone growth, bone resorption, and blood cholesterol, but is less effective in reducing cancellous bone formation and does not prevent uterine atrophy in ovariectomized (OVX) rats [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.285g/cm3 |
|---|---|
| Boiling Point | 728.2ºC at 760 mmHg |
| Melting Point | 143-147ºC |
| Molecular Formula | C28H28ClNO4S |
| Molecular Weight | 510.044 |
| Flash Point | 394.2ºC |
| Exact Mass | 509.142761 |
| PSA | 98.24000 |
| LogP | 6.81510 |
| Index of Refraction | 1.654 |
| Storage condition | -20°C Freezer |
| Water Solubility | DMSO: 28 mg/mL, soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
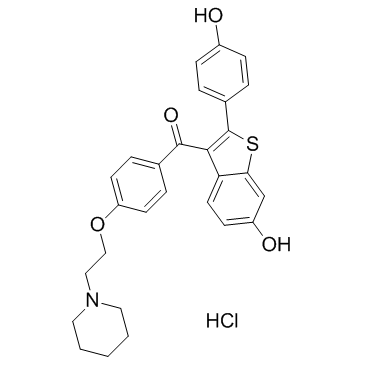
![[4-(6-acetyloxy-1-benzothiophen-2-yl)phenyl] acetate structure](https://image.chemsrc.com/caspic/336/84449-63-8.png)
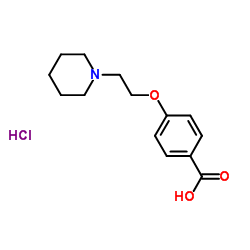


![[6-[(methylsulfonyl)oxy]-2-[4-[(methylsulfonyl)oxy]phenyl]benzo[b]thien-3-yl][4-[2-(1-pyrrolidinyl)ethoxy]phenyl]methanone hydrochloride structure](https://image.chemsrc.com/caspic/336/84449-85-4.png)

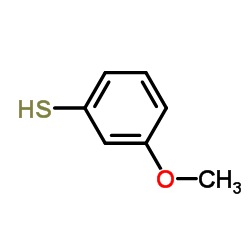
![4-Methoxy-alpha-[(3-methoxyphenyl)thio]acetophenone structure](https://image.chemsrc.com/caspic/109/63675-73-0.png)