29270-56-2
| Name | 4-fluoro-7-nitro-2,1,3-benzoxadiazole |
|---|---|
| Synonyms |
7-fluoro-4-nitrobenzofurazan
4-fluoro-7-nitrobenz-2-oxa-1,3-diazole NBD-F 7-Fluoro-4-nitrobenzo-2-oxa-1,3-diazole 4-fluoro-7-nitro-2,1,3-benzooxadiazole 4-Fluoro-7-nitro-2,1,3-benzoxadiazole MFCD00010196 4-Fluoro-7-nitrobenzofurazan (4-Fluoro-7-nitro-2,1,3-benzoxadiazole) NBD fluoride |
| Description | NBD-F is a fluorescent derivatization reagent which is originally developed for amino acid analysis. |
|---|---|
| Related Catalog | |
| In Vitro | As the percentage of the organic phase changes, the retention time of NBD-F remains relatively stable, while the retention times of the derivatization products changes. The pH of the mobile phase affects the separation of the NBD-F and the derivatization products[1]. NBD-F is a fluorescent derivatization reagent that is originally developed for amino acid analysis, and recently applied to the analysis of other amino acid derivatives such as N-methyl-D-aspartic acid and glutathione. The use of NBD-F appears to have several advantages in that the derivatization procedure is simple and its derivatives are highly stable[2]. |
| Kinase Assay | An accurately weighed quantity (0.0183 g) of NBD-F is transferred into a 1 mL centrifuge tube, dissolved in acetonitrile and made up to volume with the same solvent to produce stock solutions of 0.1 M. The solution is protected from light and stored at -20°C until analyzed. A 100 μL aliquot of mixed amino acids solution or sample supernatant, 175 μL of borate buffer solution, 200 μL of acetonitrile and 25 μL of NBD-F working solution are mixed in a 1.5 mL centrifuge tube. The well-mixed solution is allowed to react at 60°C in the water bath for 7 min, excluding light. NBD-F reacts with amino group and enables amino acids to be detected with UV detection. After cooling to room temperature, 10 μL of the solution is injected into the equilibrated HPLC system[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 308.6±45.0 °C at 760 mmHg |
| Melting Point | 52-54 °C(lit.) |
| Molecular Formula | C6H2FN3O3 |
| Molecular Weight | 183.097 |
| Flash Point | 140.4±28.7 °C |
| Exact Mass | 183.008026 |
| PSA | 84.74000 |
| LogP | 1.27 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.638 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S36-S39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~% 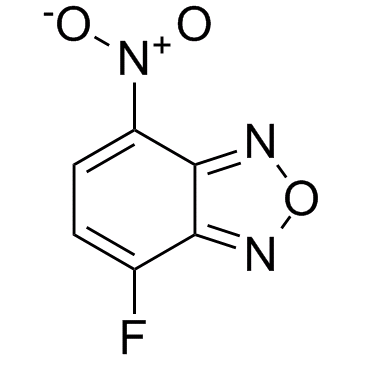
29270-56-2 |
| Literature: Jung, Michael E.; Dong, Timothy A.; Cai, Xiaolu Tetrahedron Letters, 2011 , vol. 52, # 20 p. 2533 - 2535 |
|
~% 
29270-56-2 |
| Literature: Jung, Michael E.; Dong, Timothy A.; Cai, Xiaolu Tetrahedron Letters, 2011 , vol. 52, # 20 p. 2533 - 2535 |
|
~% 
29270-56-2 |
| Literature: Jung, Michael E.; Dong, Timothy A.; Cai, Xiaolu Tetrahedron Letters, 2011 , vol. 52, # 20 p. 2533 - 2535 |
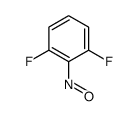
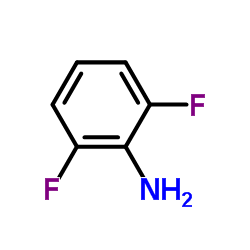
| Precursor 3 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![(2R,3R,4S,5R)-3,4,5,6-TETRAHYDROXY-2-((7-NITROBENZO[C][1,2,5]OXADIAZOL-4-YL)AMINO)HEXANAL structure](https://image.chemsrc.com/caspic/417/174844-42-9.png)