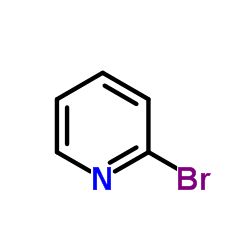
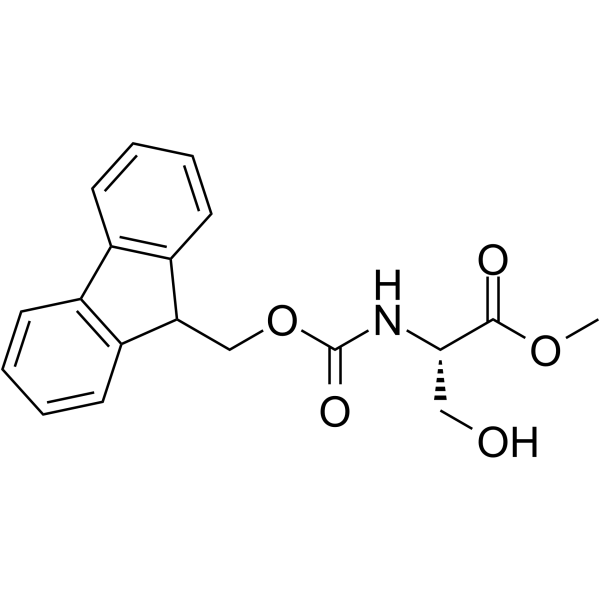
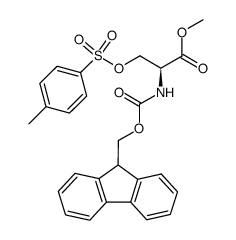
185379-40-2
| Name | Fmoc-L-2-pyridylalanine |
|---|---|
| Synonyms |
FMOC-ALA(2-PYRI)-OH
RARECHEM BK PT 0229 Fmoc-L-2-Pal-OH FMOC-ALA(2'-PYRIDYL)-OH FMOC-D-2-PYRIDYLALA RARECHEM BK PT 0232 FMOC-D-ALA(2-PYRIDYL)-OH FMOC-2'-PYRIDYL-D-ALA (2S)-2-{[(9H-fluorene-9-yl methoxy)carbonyl]amino}-3-(2-pyridinyl) propanoic acid FMOC-3-(2-PYRIDYL)-L-ALANINE FMOC-D-ALA(2-PYRI)-OH Fmoc-L-3-(2-pyridyl)alanine FMOC-3-(2-PYRIDYL)-D-ALANINE FMOC-2'-PYRIDYL-L-ALA Fmoc-L-2-Pyridylala (2S)-(N-fluorenylmethoxycarbonylamino)-3-(pyrid-2'-yl)propionic acid Fmoc-2-Pal-OH Fmoc-D-3-(2-pyridyl)-alanine N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-3-(2-pyridinyl)-L-alanine Fmoc-2-D-Pal-OH MFCD00672564 Fmoc-3-(2-Pyridyl)-Alanine Fmoc-3-(2-pyridyl)-L-Ala-OH |
| Description | (αS)-α-[[(9H-Fluoren-9-ylmethoxy)carbonyl]amino]-2-pyridinepropanoic acid is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 630.9±55.0 °C at 760 mmHg |
| Melting Point | 151ºC |
| Molecular Formula | C23H20N2O4 |
| Molecular Weight | 388.416 |
| Flash Point | 335.4±31.5 °C |
| Exact Mass | 388.142303 |
| PSA | 88.52000 |
| LogP | 3.91 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.637 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~61% 
185379-40-2 |
| Literature: Tabanella, Stefania; Valancogne, Ingrid; Jackson, Richard F W Organic and biomolecular chemistry, 2003 , vol. 1, # 23 p. 4254 - 4261 |
|
~% 
185379-40-2 |
| Literature: Organic and biomolecular chemistry, , vol. 1, # 23 p. 4254 - 4261 |
|
~% 
185379-40-2 |
| Literature: Organic and biomolecular chemistry, , vol. 1, # 23 p. 4254 - 4261 |
|
~% 
185379-40-2 |
| Literature: Organic and biomolecular chemistry, , vol. 1, # 23 p. 4254 - 4261 |
|
~% 
185379-40-2 |
| Literature: Organic and biomolecular chemistry, , vol. 1, # 23 p. 4254 - 4261 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |