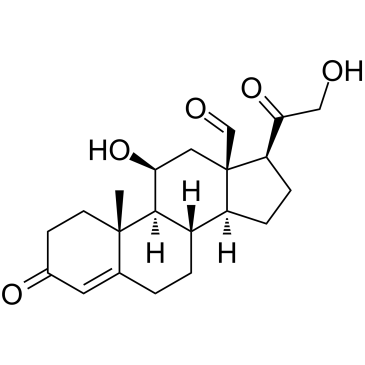
52-39-1
| Name | aldosterone |
|---|---|
| Synonyms |
11β,21-Dihydroxy-3,20-dioxo-pregn-4-en-18-al
(11β)-11,21-dihydroxy-3,20-dioxo-Pregn-4-en-18-al Aldocorten Aldocortene (11b)-11,21-dihydroxy-3,20-dioxo-pregn-4-en-18-al 11-β,21-Dihydroxy-3,20-dioxopregn-4-en-18-al Aldocortin MFCD00051136 Aldosterone (11β)-11,21-Dihydroxy-3,20-dioxopregn-4-en-18-al d-Aldosterone 18-Oxocorticosterone (11b)-11,21-Dihydroxy-3,20-dioxopregn-4-en-18-al 18-Formyl-11b,21-dihydroxy-4-pregnene-3,20-dione 11b,21-dihydroxy-3,20-dioxo-pregn-4-en-18-al 11β,21-Dihydroxypregn-4-ene-3,18,20-trione (8S,9S,10R,11S,13R,14S,17S)-11-hydroxy-17-(2-hydroxyacetyl)-10-methyl-3-oxo-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthrene-13-carbaldehyde Reichstein X Elektrocortin 4-pregnen-11,21-diol-3,20-dione-18-al EINECS 200-139-9 Electrocortin |
| Description | Aldosterone is the primary mineralocorticoid. Aldosterone is a steroid hormone, and it is synthesized and secreted in response to renin-angiotensin system activation (RAS) or high dietary potassium by the zona glomerulosa (ZG) of the adrenal cortex. Aldosterone activity is dependent by the binding and activation of the cytoplasmic/nuclear mineralocorticoid receptor (MR) at cellular level[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Aldosterone (1-1000 nM; 24 hours) inhibits interleukin-1β-stimulated nitrite production by vascular smooth muscle cells in a dose-dependent manner[3]. |
| In Vivo | Aldosterone (1 mg/Kg+1% NaCl; i.h.; once daily for 3 weeks) significantly increases systolic blood pressure (SBP), diastolic blood pressure (DBP), left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP)[4]. Aldosterone (0.72 mg/kg/day; 14 days) causes a small increase ( 14 mmHg) in blood pressure in male mice[5]. Animal Model: Forty male Wistar rats[4] Dosage: 1 mg/Kg (+1% NaCl) Administration: i.h.; once daily for 3 weeks Result: Systolic blood pressure (SBP), diastolic blood pressure (DBP), left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP) were significantly higher in aldosterone-salt-treated animals. |
| References |
[1]. Nanba K, et al. Aging and Adrenal Aldosterone Production. Hypertension. 2018 Feb;71(2):218-223. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 568.1±50.0 °C at 760 mmHg |
| Melting Point | 170-172ºC |
| Molecular Formula | C21H28O5 |
| Molecular Weight | 360.444 |
| Flash Point | 311.4±26.6 °C |
| Exact Mass | 360.193665 |
| PSA | 91.67000 |
| LogP | 0.73 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.586 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 22-24/25-36-26 |
| RIDADR | UN 1648 3 / PGII |
| WGK Germany | 3 |
| RTECS | TU4523000 |
| HS Code | 29372900 |
|
~% 
52-39-1 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1423,1436 Experientia, , vol. 12, p. 50 |
|
~% 
52-39-1 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1423,1436 |
|
~% 
52-39-1 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1423,1436 |
|
~% 
52-39-1 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1423,1436 |
|
~% 
52-39-1 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1423,1436 |
|
~% 
52-39-1 |
| Literature: Steroids, , vol. 36, # 5 p. 601 - 609 |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 29372900 |
|---|