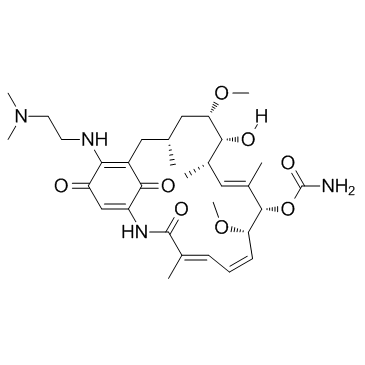
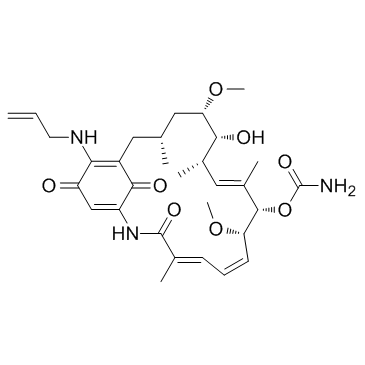
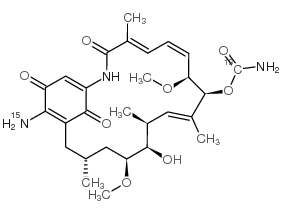
30562-34-6
| Name | geldanamycin |
|---|---|
| Synonyms |
GELDANAMYCIN,STREPTOMYCES HYGROSCOPICUS
Streptomyces hygroscopicus GELDANAMYCIN FROM STRE (4Z,6Z,8S,9R,10Z,12R,14R,16R)-13-Hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate Geldanamycin MFCD00274570 Geldanamycin from Streptomyces hygroscopicus (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-Hydroxy-8,14,19-trimethoxy-4,10,12,16-tetramethyl-3,20,22-trioxo-2-azabicyclo[16.3.1]docosa-1(21),4,6,10,18-pentaen-9-yl carbamate Geldanamycin (9CI) |
| Description | Geldanamycin is a Hsp90 inhibitor with antimicrobial activity against many Gram-positive and some Gram-negative bacteria. |
|---|---|
| Related Catalog | |
| Target |
HSP90:1.2 μM (Kd) |
| In Vitro | Geldanamycin significantly delays and reduces viperin expression, indicating that IRF3 is involved in viperin induction in RAW264.7 cells[1]. Geldanamycin (GA), a benzoquinone ansamycin, protected against neuronal injury induced by oxygen-glucose deprivation (OGD)/zVAD treatment in cultured primary neurons. More importantly, Geldanamycin decreases RIP1 protein level in a time and concentration-dependent manner. Geldanamycin also decreases the Hsp90 protein level, which causes instability of RIP1 protein, resulting in decreased RIP1 protein level but not RIP1 mRNA level after Geldanamycin treatment[2]. Geldanamycin (GA) is identified as the first natural product inhibitor of Hsp90 that binds to the N-terminal ATPase domain of Hsp90 to inhibit its chaperone function, and significantly induces tumor cell death via an apoptotic mechanism[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 783.9±60.0 °C at 760 mmHg |
| Melting Point | 255 °C |
| Molecular Formula | C29H40N2O9 |
| Molecular Weight | 560.636 |
| Flash Point | 427.9±32.9 °C |
| Exact Mass | 560.273376 |
| PSA | 163.48000 |
| LogP | 2.00 |
| Vapour Pressure | 0.0±6.2 mmHg at 25°C |
| Index of Refraction | 1.559 |
| Storage condition | −20°C |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T: Toxic; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-27-36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | LX8920000 |
| HS Code | 2933990090 |
| Precursor 2 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![(3S,5S,6S)-3-[(1S,3R)-1-methoxy-3-methyl-4-(5-nitro-2,3,6-trimethoxy-phenyl)-butyl]-5,6-bis-(4-methoxy-phenyl)-[1,4]dioxan-2-one structure](https://image.chemsrc.com/caspic/349/326606-26-2.png)