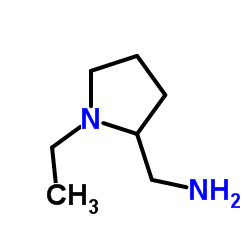
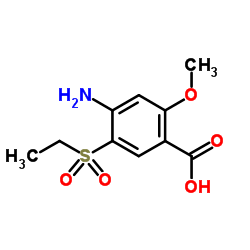
71675-85-9
| Name | amisulpride |
|---|---|
| Synonyms |
Amisulpiride
Aminosultopride Socian 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide Amisulpride 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamide Amisulpridum [INN-Latin] amisulpridum 4-amino-N-[(1-ethyl-2-pyrrolidin-yl)methyl]-5-(ethyl-sulfonyl)-2--methoxybenzamide MFCD00866691 Deniban EINECS 275-831-7 Solian UNII:8110R61I4U 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-o-anisamide amisulprida 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide |
| Description | Amisulpride is a dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.8 nM (D2 receptor), 3.2 nM (D3 receptor)[1] |
| In Vitro | Amisulpride is an atypical dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. Amisulpride (100 nM) inhibits quinpirole-elicited [3H]thymidine incorporation with an IC50 value of 22±3 nM (n=3). Amisulpride slightly but significantly increases [3H]dopamine release from slices of the rat striatum (S2/S1=0.88±0.04 under control conditions, n=6; 1.04±0.08 in the presence of 100 nM Amisulpride,n=4; P<0.05) and opposes the inhibitory effects of 7-OH-DPAT in both brain areas[1]. |
| In Vivo | Only the highest dose of Amisulpride (100 mg/kg) significantly reduces dopamine levels in the striatum or limbic system. Amisulpride significantly increases the synthesis of dopamine in the rat striatum and limbic system at doses of 20 and 100 mg/kg. Amisulpride (0.5 to 75 mg/kg) fails to provoke an additional increase in dopa accumulation in the striatum but slightly accelerates, at 75 mg/kg, dopamine synthesis in the limbic system. In comparison with vehicle-treated controls, Amisulpride (10 mg/kg) increases extracellular dopamine levels. The administration of Amisulpride (0.5 to 15 mg/kg s.c.) provokes a time- and dose-dependent increase in the stimulation-evoked dopamine release. Amisulpride decreases striatal ACh levels significantly at 30 and 100 mg/kg (87.5% and 56.3% of control levels, respectively)[1]. In both acute study, Amisulpride (70 mg/kg, p.o.) significantly increases the duration of swimming behavior [F(3,28)=45.90, p<0.01][2]. |
| Cell Assay | The functional effects of Amisulpride at the dopamine D3 receptor subtype are assessed. Briefly, the mitogenic response elicited in NG108-15 neuroblastoma-glioma cells stably transfected with human dopamine D3 receptor cDNA by the addition of 10 nM quinpirole in the presence of 1 μM forskolin is quantified by the incorporation of [3H]thymidine. Antagonism of quinpirole-induced mitogenesis is measured in the presence of increasing (0.1 to 100 nM) concentrations of Amisulpride[1]. |
| Animal Admin | A total of 64 male Swiss albino mice weighing between 20 to 30 g are used. The animals are fed with standard pellet diet and water ad libitum. The mice are divided in different groups (n=8 in each group) and drug administration is done as follows: Group 1 (control): distilled water (1 mL/kg) 23.5, 5 and 1 h before the test. Group 3 (Amisulpride): Amisulpride (70 mg/kg) 23.5, 5 and 1 h before the test[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 558.9±50.0 °C at 760 mmHg |
| Melting Point | 124-128ºC |
| Molecular Formula | C17H27N3O4S |
| Molecular Weight | 369.479 |
| Flash Point | 291.8±30.1 °C |
| Exact Mass | 369.172241 |
| PSA | 110.11000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.546 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥5 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CV2308701 |
| HS Code | 2933990090 |
|
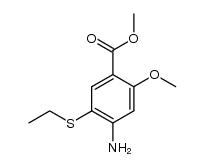
~70% 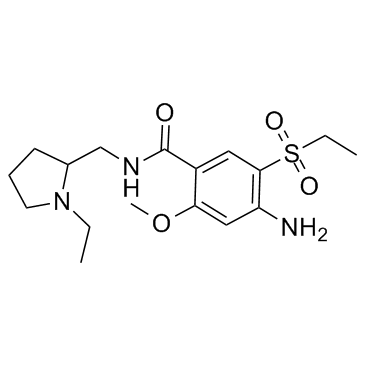
71675-85-9 |
| Literature: LUPIN LIMITED; PAGHDAR, Dinesh, Jayntibhai; KOLEKAR, Mahesh, Ramkumar; DESHPANDE, Tushar, Nandkumar; PATIL, Suryaprakash, Pandurang; CHAVAN, Yuvraj, Atmaram; RAY, Purna, Chandra; SINGH, Girij, Pal Patent: WO2011/158084 A1, 2011 ; Location in patent: Page/Page column 14-15 ; |
|
~99% 
71675-85-9 |
| Literature: Societe d'Etudes Scientifiques et Industrielles de l'Ile de-France Patent: US4294828 A1, 1981 ; |
|
~61% 
71675-85-9 |
| Literature: Societe d'Etudes Scientifiques et Industrielles de l'Ile de-France Patent: US4294828 A1, 1981 ; |
|
~% 
71675-85-9 |
| Literature: WO2011/158084 A1, ; |
|
~% 
71675-85-9 |
| Literature: WO2011/158084 A1, ; |
|
~% 
71675-85-9 |
| Literature: WO2011/158084 A1, ; |
|
~% 
71675-85-9 |
| Literature: WO2011/158084 A1, ; |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |