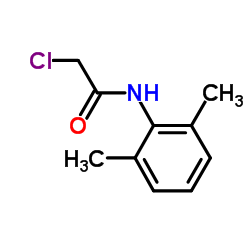
77191-36-7
| Name | N-(2,6-Dimethylphenyl)-2-(2-oxopyrrolidin-1-yl)acetamide |
|---|---|
| Synonyms |
N-(2,6-dimethylphenyl)-2-(2-oxopyrrolidin-1-yl)acetamide
Nefiracetam MFCD00209882 Dmmpa 2-(2-Oxo-1-pyrrolidinyl)-N-(2,6-dimethylphenyl)acetamide N-(2,6-Dimethylphenyl)-2-(2-oxo-1-pyrrolidinyl)acetamide |
| Description | Nefiracetam is a GABAergic, cholinergic, and monoaminergic neuronal systems enhancer for Ro 5-4864-induced convulsions.Target: GABA ReceptorNefiracetam induces a short-term depression of ACh-evoked currents at submicromolar concentrations (0.01-0.1 μM) and a long-term enhancement of the currents at micromolar concentrations (1-10 μM). Nefiracetam interacts with PKA and PKC pathways, which may explain a cellular mechanism for the action of cognition-enhancing agents. Lower (submicromolar) concentrations of the nootropic Nefiracetam reduces ACh-evoked currents to 30% (0.01 μM) and 38% (0.1 μM) of control after a 10-minute treatment [1].Nefiracetam administered orally inhibits Ro 5-4864-induced convulsions in EL mice. Nefiracetam also efficiently inhibits Ro 5-4864-induced convulsions in DDY mice at doses higher than 10 mg/kg [2]. Nefiracetam administered daily 1 hour before each training session facilitates the acquisition process of the avoidance response [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 458.5±33.0 °C at 760 mmHg |
| Melting Point | 151-155°C |
| Molecular Formula | C14H18N2O2 |
| Molecular Weight | 246.305 |
| Flash Point | 231.1±25.4 °C |
| Exact Mass | 246.136826 |
| PSA | 49.41000 |
| LogP | 1.53 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.594 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| RTECS | UX9655650 |
| HS Code | 2933790090 |
|
~87% 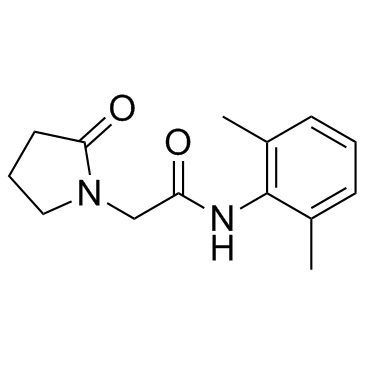
77191-36-7 |
| Literature: Daiichi Pharmaceutical Co., Ltd. Patent: US5461157 A1, 1995 ; |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |