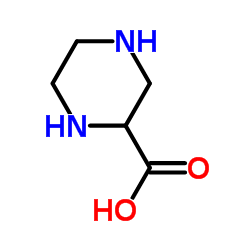
100828-16-8
| Name | (RS)-3-(2-Carboxypiperazin-4-yl)-propyl-1-phosphonicacid |
|---|---|
| Synonyms |
3-2-Cpp
DL-CPP |
| Description | (RS)-CPP ((±)-CPP) is a potent and selective NMDA antagonist. (RS)-CPP inhibits central neuron responses, and has anticonvulsant activity[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.408g/cm3 |
|---|---|
| Boiling Point | 546.7ºC at 760mmHg |
| Molecular Formula | C8H17N2O5P |
| Molecular Weight | 252.20 |
| Flash Point | 284.4ºC |
| Exact Mass | 252.08800 |
| PSA | 119.91000 |
| Vapour Pressure | 2.14E-13mmHg at 25°C |
| Index of Refraction | 1.53 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 22-26-36 |
| RIDADR | NONH for all modes of transport |
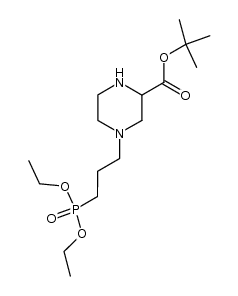
|
~76% 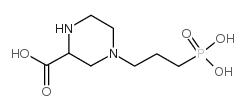
100828-16-8 |
| Literature: Demaine; Smith Synthesis, 1992 , # 11 p. 1065 - 1067 |
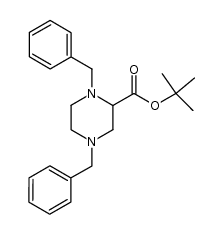
|
~% 
100828-16-8 |
| Literature: British Technology Group Limited Patent: US5399693 A1, 1995 ; |
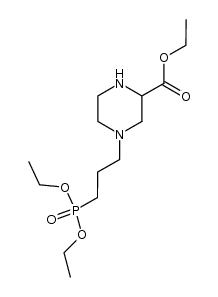
|
~% 
100828-16-8 |
| Literature: Demaine; Smith Synthesis, 1992 , # 11 p. 1065 - 1067 |
|
~75% 
100828-16-8 |
| Literature: Demaine; Smith Synthesis, 1992 , # 11 p. 1065 - 1067 |
|
~% 
100828-16-8 |
| Literature: Pevarello, P.; Scappi, G.; Varasi, M. Organic Preparations and Procedures International, 1994 , vol. 26, # 3 p. 366 - 370 |
|
~% 
100828-16-8 |
| Literature: Demaine; Smith Synthesis, 1992 , # 11 p. 1065 - 1067 |
|
~% 
100828-16-8 |
| Literature: Pevarello, P.; Scappi, G.; Varasi, M. Organic Preparations and Procedures International, 1994 , vol. 26, # 3 p. 366 - 370 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |