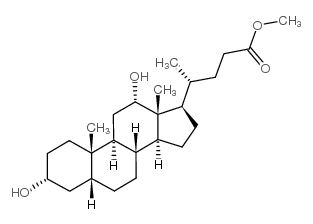
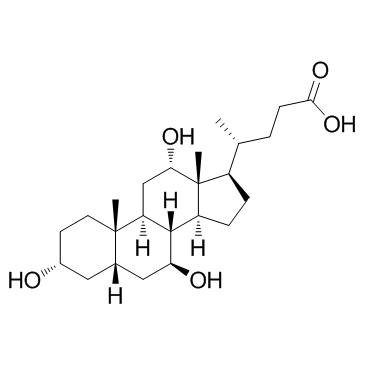
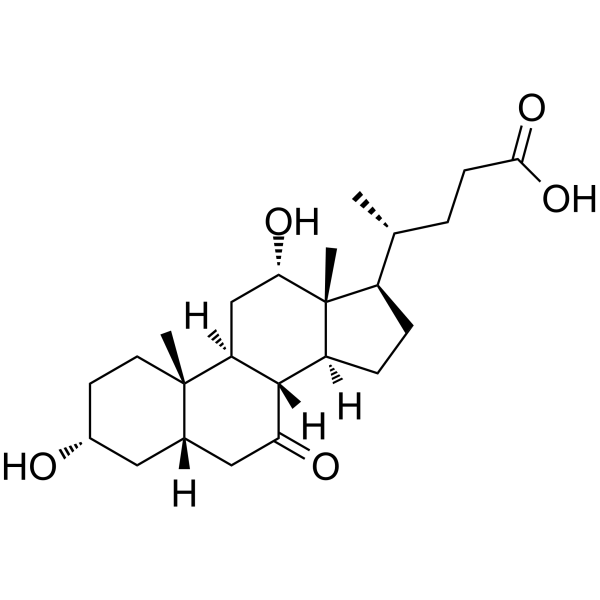
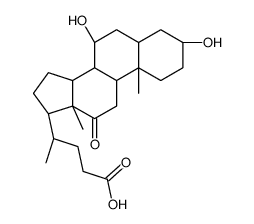
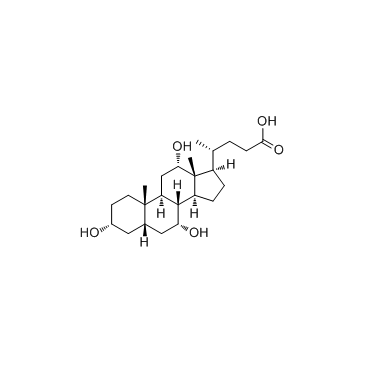
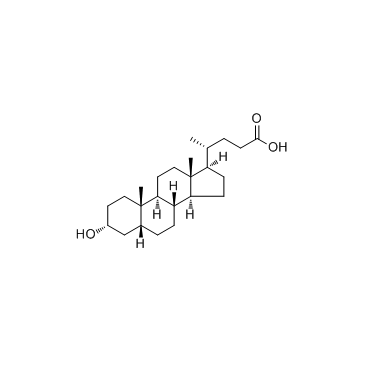
434-13-9
| Name | lithocholic acid |
|---|---|
| Synonyms |
17b-(1-Methyl-3-carboxypropyl)etiocholan-3a-ol
(3α,5β)-3-Hydroxycholan-24-oic acid 3-α-Hydroxy-5-β-cholanic acid EINECS 207-099-1 4-10-00-00785 (Beilstein Handbook Reference) 5β-Cholanic Acid-3α-ol Lithocholic acid Lithocolic acid 3α-Hydroxy-5β-cholanic Acid MFCD00003682 (3a,5b)-3-hydroxycholan-24-oic acid 3a-Hydroxycholanic Acid (4R)-4-[(3R,5R,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid (3a,5b)-3-hydroxy-cholan-24-oic acid (4R)-4-[(3R,5R,8R,9S,10S,13R,14S,17R)-3-Hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid 3a-Hydroxy-5b-cholan-24-oic Acid Cholan-24-oic acid, 3-hydroxy-, (3-α,5-β)- (9CI) 3α-hydroxy-5β-cholan-24-oic acid |
| Description | Lithocholic acid is a toxic secondary bile acid, causes intrahepatic cholestasis, has tumor-promoting activity.Target: OthersLithocholic acid has been used in a study to assess cholestasis and its action on several organs and tissues in rats. It has also been used in a study to investigate the regulation of hepatic phospholipid and bile acid homeostasis through SMAD3 activation by TGFβ. It has been implicated in human and experimental animal carcinogenesis. Preliminary in vitro research suggests that LCA selectively kills neuroblastoma cells, while sparing normal neuronal cells and is cytotoxic to numerous other malignant cell types at physiologically relevant concentrations. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 511.0±23.0 °C at 760 mmHg |
| Melting Point | 183-188 °C(lit.) |
| Molecular Formula | C24H40O3 |
| Molecular Weight | 376.573 |
| Flash Point | 276.9±19.1 °C |
| Exact Mass | 376.297760 |
| PSA | 57.53000 |
| LogP | 6.70 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.528 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F+ |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | FZ2275000 |
| Precursor 8 | |
|---|---|
| DownStream 9 | |

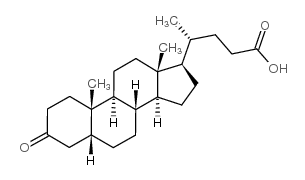
![(R)-4-((3R,5R,8R,9S,10S,13R,14S,17R)-3-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)-1-(phenanthridin-5(6H)-yl)pentan-1-one structure](https://image.chemsrc.com/caspic/030/86931-55-7.png)
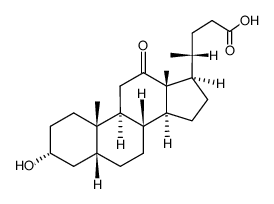
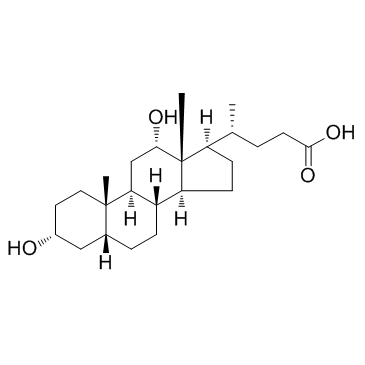
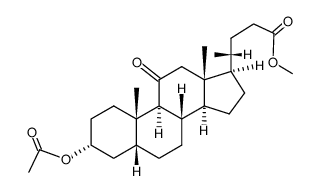
![methyl 3alpha-[(ethoxycarbonyl)oxy]-7,12-dioxo-5beta-cholan-24-oate structure](https://image.chemsrc.com/caspic/052/21059-42-7.png)