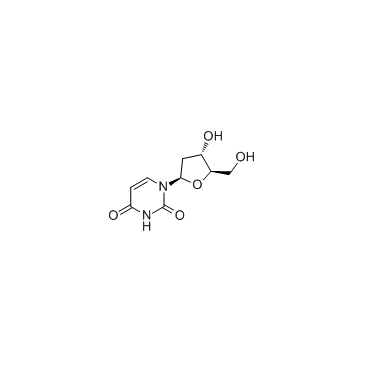
5580-20-1
| Name | 2(1H)-Pyrimidinone, 1-(2-deoxy-.β.-D-erythro-pentofuranosyl)-3,4-dihydro-4-thioxo |
|---|---|
| Synonyms |
desoxysesbanine
4-thiodeoxyuridine 2'-deoxy-4-thiouridine Deoxythiouridine DEOXYSESBANINE 4-thio-2'-deoxyuridine |
| Description | 4-Thio-2’-deoxyuridine is a purine nucleoside analog. Purine nucleoside analogs have broad antitumor activity targeting indolent lymphoid malignancies. Anticancer mechanisms in this process rely on inhibition of DNA synthesis, induction of apoptosis, etc[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.59g/cm3 |
|---|---|
| Melting Point | 169 °C |
| Molecular Formula | C9H12N2O4S |
| Molecular Weight | 244.27 |
| Exact Mass | 244.05200 |
| PSA | 119.57000 |
| Index of Refraction | 1.697 |
|
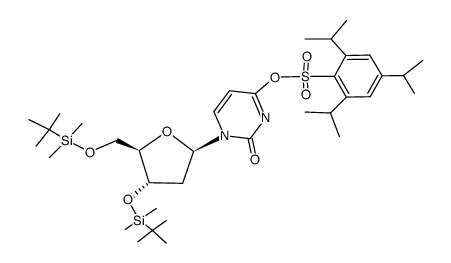
~78% 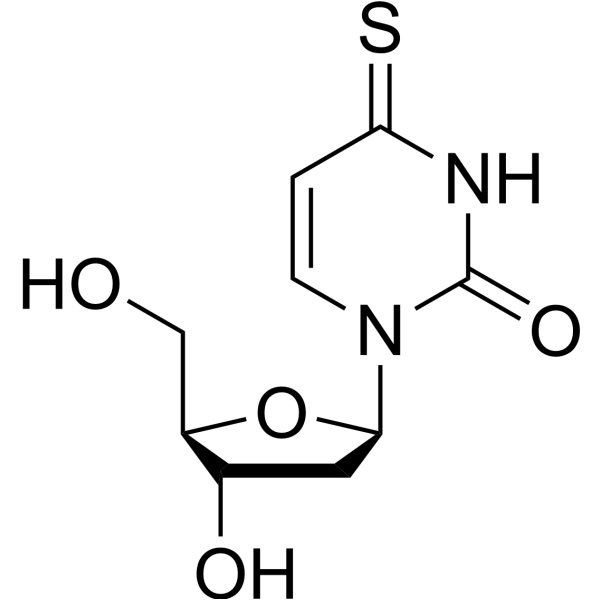
5580-20-1 |
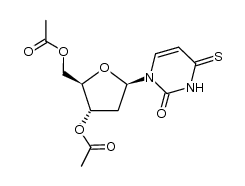
| Literature: Coleman, Robert S.; Kesicki, Edward A. Journal of the American Chemical Society, 1994 , vol. 116, # 26 p. 11636 - 11642 |
|
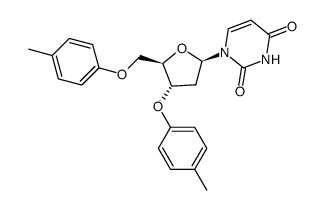
~% 
5580-20-1 |
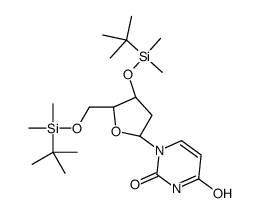
| Literature: Toppin, Charles R.; Pauly, Gary T.; Devanesan, Prabu; Kryak, David; Bobst, Albert M. Helvetica Chimica Acta, 1986 , vol. 69, p. 345 - 349 |
|
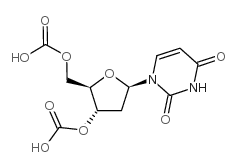
~% 
5580-20-1 |
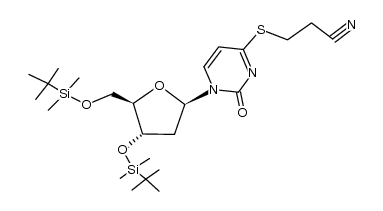
| Literature: Coleman; Siedlecki Tetrahedron Letters, 1991 , vol. 32, # 26 p. 3033 - 3034 |
|
~% 
5580-20-1 |
| Literature: Coleman; Siedlecki Tetrahedron Letters, 1991 , vol. 32, # 26 p. 3033 - 3034 |
|
~% 
5580-20-1 |
| Literature: Coleman; Siedlecki Tetrahedron Letters, 1991 , vol. 32, # 26 p. 3033 - 3034 |
|
~% 
5580-20-1 |
| Literature: Zhang, Xiaohui; Xu, Yao-Zhong Molecules, 2011 , vol. 16, # 7 p. 5655 - 5664 |
|
~% 
5580-20-1 |
| Literature: Ruda, Gian Filippo; Nguyen, Corinne; Ziemkowski, Przemyslaw; Felczak, Krzysztof; Kasinathan, Ganasan; Musso-Buendia, Alexander; Sund, Christian; Zhou, Xiao Xiong; Kaiser, Marcel; Ruiz-Perez, Luis M.; Brun, Reto; Kulikowski, Tadeusz; Johansson, Nils Gunnar; Gonzalez-Pacanowska, Dolores; Gilbert, Ian H. ChemMedChem, 2011 , vol. 6, # 2 p. 309 - 320 |
|
~% 
5580-20-1 |
| Literature: Ruda, Gian Filippo; Nguyen, Corinne; Ziemkowski, Przemyslaw; Felczak, Krzysztof; Kasinathan, Ganasan; Musso-Buendia, Alexander; Sund, Christian; Zhou, Xiao Xiong; Kaiser, Marcel; Ruiz-Perez, Luis M.; Brun, Reto; Kulikowski, Tadeusz; Johansson, Nils Gunnar; Gonzalez-Pacanowska, Dolores; Gilbert, Ian H. ChemMedChem, 2011 , vol. 6, # 2 p. 309 - 320 |
|
~% 
5580-20-1 |
| Literature: Visser; Elsinga; Vaalburg Journal of Labelled Compounds and Radiopharmaceuticals, 1999 , vol. 42, # SUPPL. 1 p. S614-S616 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |