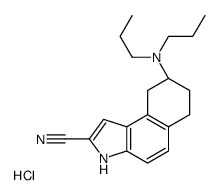
149654-41-1
| Name | (8R)-8-(dipropylamino)-6,7,8,9-tetrahydro-3H-benzo[e]indole-2-carbonitrile |
|---|---|
| Synonyms |
U-92,016-A
(+)-R)-2-cyano-N,N-dipropyl-8-amino-6,7,8,9-tetrahydro-3H-benz[e]indole |
| Description | U92016A hydrochloride is a potent, metabolically stable, orally acitive 5-HT1A receptor agonist with an exceptionally high degree of intrinsic activity[1][2]. U92016A hydrochloride binds with high affinity to human 5-HT1A receptors expressed in Chinese hamster ovary cells (Ki=0.2 nM)[2]. |
|---|---|
| Related Catalog | |
| Target |
5-HT1A Receptor:0.2 nM (Ki) |
| In Vitro | U92016A (U-92016A) is selective for the 5-HT1A receptor over other biogenic amine receptors. U92016A decreases the Forskolin-induced increase in cyclic AMP synthesis and has an intrinsic activity of 0.82 relative to 5-HT in Chinese hamster ovary cells expressing the human 5HT1A receptor[2]. |
| In Vivo | U92016A (U-92016A) potently decreases rectal temperature in mice. U92016A also elicits the 5-HT-mediated syndrome in rats and results in a dose-related decrease in 5-hydroxytryptophan accumulation. U92016A also decreases arterial blood pressure in spontaneously hypertensive rats and inhibits sympathetic nerve activity in cats. U92016A displays excellent potency and a long duration of action. U92016A also inhibits the firing of dorsal raphe 5-HT neurons and is active in two social interaction assays. The p.o. bioavailability of U92016A is 45%[2]. |
| References |
| Molecular Formula | C19H26ClN3 |
|---|---|
| Molecular Weight | 331.88300 |
| Exact Mass | 331.18200 |
| PSA | 42.82000 |
| LogP | 4.82088 |