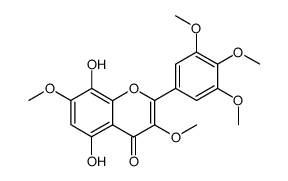
21634-52-6
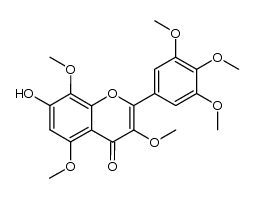
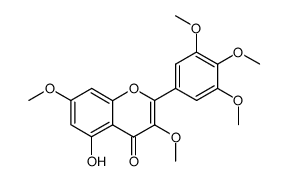
| Name | 3,5,7,8-Tetramethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one |
|---|---|
| Synonyms |
3,5,7,8-Tetramethoxy-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one
3,5,7,7-tetrachloro-hept-2-ene 2-Heptene,3,5,7,7-tetrachloro |
| Description | Hibiscetin heptamethyl ether is a natural product isolated from various medicinal plants[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 621.5±55.0 °C at 760 mmHg |
| Molecular Formula | C22H24O9 |
| Molecular Weight | 432.421 |
| Flash Point | 269.2±31.5 °C |
| Exact Mass | 432.142029 |
| PSA | 94.82000 |
| LogP | 1.65 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.576 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2914509090 |
|
~% 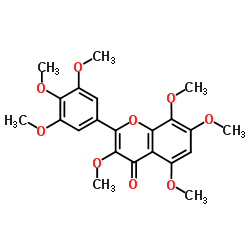
21634-52-6 |
| Literature: Horie, Tokunaru; Tsukayama, Masao; Kawamura, Yasuhiko; Yamamoto, Shigeo Phytochemistry (Elsevier), 1988 , vol. 27, # 5 p. 1491 - 1496 |
|
~% 
21634-52-6 |
| Literature: Rao; Seshadri Proceedings - Indian Academy of Sciences, Section A, 1948 , # 27 p. 104,109 |
|
~% 
21634-52-6 |
| Literature: Rao et al. Proceedings - Indian Academy of Sciences, Section A, 1944 , # 19 p. 88,91 |
|
~% 
21634-52-6 |
| Literature: Rao et al. Proceedings - Indian Academy of Sciences, Section A, 1944 , # 19 p. 88,91 |
|
~% 
21634-52-6 |
| Literature: Rao; Seshadri Proceedings - Indian Academy of Sciences, Section A, 1947 , # 25 p. 444,445 |
|
~% 
21634-52-6 |
| Literature: Rao; Seshadri Proceedings - Indian Academy of Sciences, Section A, 1947 , # 25 p. 444,445 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |