489-35-0
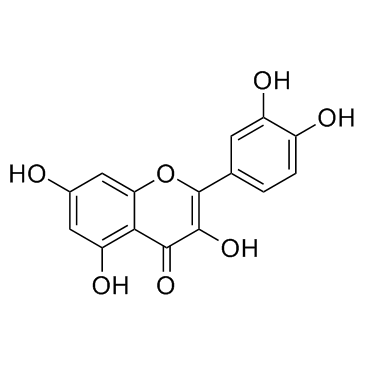
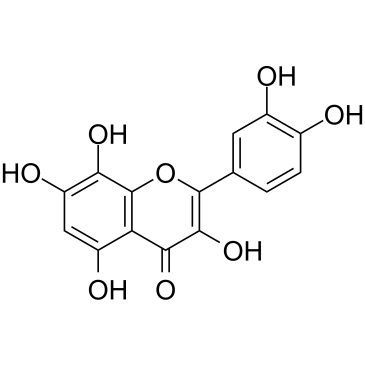
| Name | gossypetin |
|---|---|
| Synonyms |
3,5,7,8,3',4'-hexahydroxyflavone
3,3',4',5,7,8-Hexahydroxyflavone 3,5,7,8,3',4'-hexahydroxyflavon Equisporol Articulatidin 8-hydroxyquercetin 2-(3,4-dihydroxyphenyl)-3,5,7,8-tetrahydroxychromen-4-one 5,7,8,3',4'-pentahydroxyflavonol Gossypetin |
| Description | Gossypetin is a hexahydroxylated flavonoid and is a potent mitogen-activated protein kinase kinase (MKK)3 and MKK6 inhibitor with strongly attenuates the MKK3/6-p38 signaling pathway, has various pharmacological activities, including antioxidant, antibacterial and anticancer activities[1]. |
|---|---|
| Related Catalog | |
| Target |
p38 MAP kinase |
| In Vitro | Gossypetin (20-60 μM; 48 hours; KYSE30, KYSE450 and KYSE510 cells) treatment significantly inhibits anchorage-dependent esophageal cancer cell growth in dose dependent manner. Gossypetin strongly suppresses anchorage-independent cell growth in esophageal cancer cells[1]. Gossypetin (60 μM; 3 hours; KYSE30 and KYSE410 cells) treatment strongly inhibits p38 activity in a dose-dependent manner and confirms that Gossypetin directly suppresses MKK3 or MKK6 activity[1]. Gossypetin (20-40 μM; 48 hours; KYSE450 and KYSE510 cells) treatment reduces S phase and induces G2 phase cell cycle arrest in a dose-dependent manner[1]. Gossypetin (20-40 μM; 72 hours; esophageal cancer cells) treatment induces intrinsic apoptosis of esophageal cancer cells[1]. Cell Proliferation Assay[1] Cell Line: KYSE30, KYSE450 and KYSE510 cells Concentration: 20 μM, 40 μM, 60 μM Incubation Time: 48 hours Result: Anchorage-dependent esophageal cancer cell growth was significantly inhibited. Western Blot Analysis[1] Cell Line: KYSE30 and KYSE410 ccells Concentration: 60 μM Incubation Time: 3 hours Result: p38 activity was strongly inhibited in a dose-dependent manner. Cell Cycle Analysis[1] Cell Line: KYSE450 and KYSE510 cells Concentration: 20 μM, 40 μM Incubation Time: 48 hours Result: Reduced S phase and induces G2 phase cell cycle arrest in a dose-dependent manner. Apoptosis Analysis[1] Cell Line: Esophageal cancer cells Concentration: 20 μM, 40 μM Incubation Time: 72 hours Result: Induced apoptosis of esophageal cancer cells. |
| In Vivo | Gossypetin (100 mg/kg; oral administration; 5 times per week; for 21 days; severe combined immunodeficiency (SCID) female mice) treatment significantly decreases the volume of esophageal tumor growth and without significant loss of body weight. The expression of Ki67 is significantly decreased by Gossypetin. There are no obvious morphological differences between tissues from treated or untreated mic. The phosphorylation of p38, the direct downstream protein of MKK3/6 strongly inhibited in the Gossypetin-treated group[1]. Animal Model: Severe combined immunodeficiency (SCID) female mice (6-9 weeks old) injection with esophageal cancer tissue[1] Dosage: 100 mg/kg Administration: Oral administration; 5 times per week; for 21 days Result: Suppressed patient-derived esophageal xenograft tumor growth in an in vivo mouse model. |
| References |
| Density | 1.912 g/cm3 |
|---|---|
| Boiling Point | 679.3ºC at 760 mmHg |
| Melting Point | 302-304ºC |
| Molecular Formula | C15H10O8 |
| Molecular Weight | 318.23500 |
| Flash Point | 260.6ºC |
| Exact Mass | 318.03800 |
| PSA | 151.59000 |
| LogP | 1.69360 |
| Vapour Pressure | 4.49E-19mmHg at 25°C |
| Index of Refraction | 1.863 |
|
~% 
489-35-0 |
| Literature: Halbwirth, Heidrun; Stich, Karl Phytochemistry, 2006 , vol. 67, # 11 p. 1080 - 1087 |
|
~% 
489-35-0 |
| Literature: Morikawa, Toshio; Xie, Haihui; Wang, Tao; Matsuda, Hisashi; Yoshikawa, Masayuki Chemical and Pharmaceutical Bulletin, 2008 , vol. 56, # 10 p. 1438 - 1444 |
|
~% 
489-35-0 |
| Literature: Hussein, Sahar A.M.; Hashem, Amani N.M.; Seliemb, Mohammed A.; Lindequist, Ulrike; Nawwar, Mahmoud A.M. Phytochemistry, 2003 , vol. 64, # 4 p. 883 - 889 |
|
~% 
489-35-0 |
| Literature: Oliverio et al. Gazzetta Chimica Italiana, 1948 , vol. 78, p. 363,370 |