10605-02-4
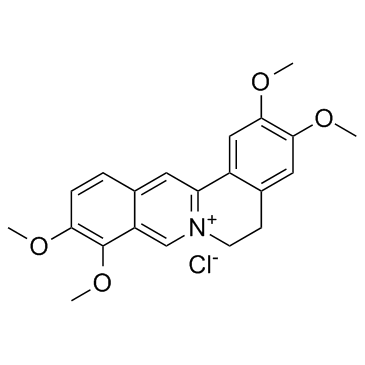
| Name | 2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium,chloride |
|---|---|
| Synonyms |
Dibenzo[a,g]quinolizinium, 5,6-dihydro-2,3,9,10-tetramethoxy-, chloride (1:1)
Palmatine chloride GNF-PF-4086 2,3,9,10-Tetramethoxy-5,6-dihydroisoquinolino[3,2-a]isoquinolinium chloride Palmatine chloride,Palmatine hydrochloride Palmatinehydrochloride 2,3,9,10-Tetramethoxy-5,6-dihydroisoquino[3,2-a]isoquinolinium chloride Prestwick_374 Palmatine (chloride) |
| Description | Palmatine chloride an isoquinoline alkaloid, is an important medicinal herbal extract with diverse pharmacological and biological properties. IC50 value:Target:In vitro: Experimental set examined the influence of palmatine on osteoblast-like cells in vitro. In the culture supernatant of MC3T3-E1 cells, RANKL and OPG levels were significantly reduced by palmatine addition [1]. In vivo: The first experimentaI set was designed to histologically and biochemically examine mice randomly divided into four groups: sham-operated, OVX, and OVX-palmatine intake groups (1 mg/kg and 10 mg/kg). In palmatine-treated mice, RANKL and OPG expression decreased [1]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 205 °C |
|---|---|
| Molecular Formula | C21H22ClNO4 |
| Molecular Weight | 387.857 |
| Exact Mass | 387.123749 |
| PSA | 40.80000 |
| LogP | 0.38880 |
| Vapour Pressure | 6.6E-15mmHg at 25°C |
| Index of Refraction | 1.624 |
| Risk Phrases | 20/22-36/38 |
|---|---|
| Safety Phrases | 24/45 |
| HS Code | 2933990090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |