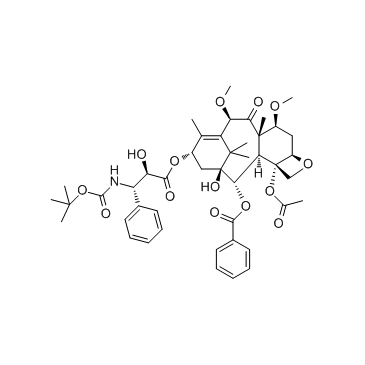
32981-86-5
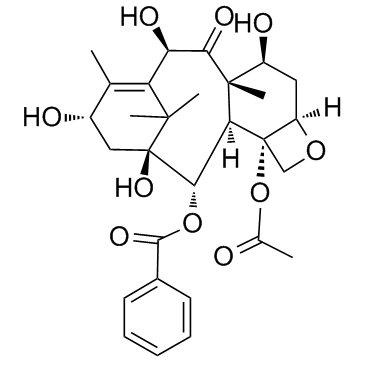
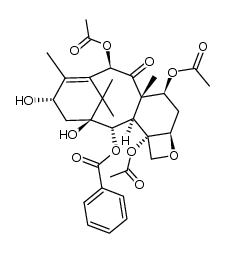
| Name | 10-deacetylbaccatin III |
|---|---|
| Synonyms |
10-Dab
10-DAB (10-Deacetylbaccatin) MFCD00132913 EINECS 418-680-6 (2α,5β,7β,10α,13α)-4-Acetoxy-1,7,10,13-tetrahydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate (2α,5β,7β,10β,13α)-4-(acetyloxy)-1,7,10,13-tetrahydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate 10-Deacetylbaccatine 10-deacetylbaccatin11 10-DAB-III 10DBA III 10-Deacetyl 10-DEACETYLBACCATIN 10-Deacetylbaccatin-III 10-desacetylbaccatin III 10-Deacetylbaccatin III DESACETYLBACCATINE Docetaxel impurity E 7,11-Methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one, 12b-(acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,6,9,11-tetrahydroxy-4a,8,13,13-tetramethyl-, (2aR,4S,4aS,6S,9S,11S,12S,12aR,12bS)- (2α,5β,7β,10β,13α)-4-Acetoxy-1,7,10,13-tetrahydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate 7,11-Methano-5H-cyclodeca[3,4]benz[1,2-b]oxet-5-one, 12b-(acetyloxy)-12-(benzoyloxy)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-dodecahydro-4,6,9,11-tetrahydroxy-4a,8,13,13-tetramethyl-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)- |
| Description | 10-Deacetylbaccatin-III is an intermediate for taxol analog preparations. IC50 value:Target: Taxols have exhibit antitumor agents. Several of these taxols can be synthesized from 10- Deacetylbaccatin-III. 10-Deacetylbaccine III is the fifth intermediate of paclitaxel biosynthesis. The biosynthetic pathway consists of approximately 20 enzymatic steps but is not fully elucidated. 10-Deacetylbaccine III is an antineoplastic agent and an anti-cancer intermediate. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 716.8±60.0 °C at 760 mmHg |
| Melting Point | 231-236 °C |
| Molecular Formula | C29H36O10 |
| Molecular Weight | 544.590 |
| Flash Point | 233.5±26.4 °C |
| Exact Mass | 544.230835 |
| PSA | 159.82000 |
| LogP | 3.51 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.624 |
| Water Solubility | INSOLUBLE |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/24/25 |
| Safety Phrases | S45-S38-S36/37/39-S28A |
| RIDADR | 1544 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 7 | |
|---|---|
| DownStream 10 | |


![(3aS,5S,7aS,10aS,11S,12R,12aS)-6,9,9,11,13,13-hexamethyl-2-oxo-5-((triisopropylsilyl)oxy)-5,7a,10a,11,12,12a-hexahydro-4H-3a,7-methanocyclodeca[1,2-d:5,6-d']bis([1,3]dioxole)-12-carbaldehyde structure](https://image.chemsrc.com/caspic/032/187960-83-4.png)
![2-((3aS,5S,8S,9S,10S,11R,11aS)-8,9-dihydroxy-6,10,12,12-tetramethyl-2-oxo-5-((triisopropylsilyl)oxy)-5,8,9,10,11,11a-hexahydro-4H-3a,7-methanocyclodeca[d][1,3]dioxol-11-yl)acetaldehyde structure](https://image.chemsrc.com/caspic/456/187960-85-6.png)
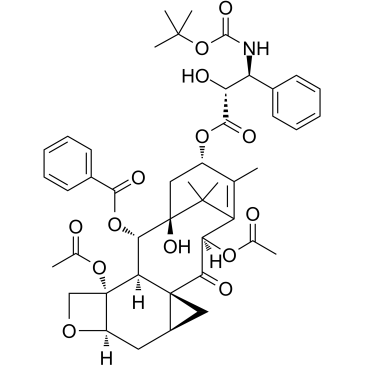
![13-{[(3-t-Boc)-2,2-dimethyl-4S-phenyl-1,3-oxazolidin-5R-yl]formyl}-7-O-(2,2,2-trichloroethyl)oxy]carbonyl) Baccatin III structure](https://image.chemsrc.com/caspic/107/143527-73-5.png)
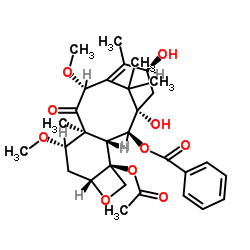
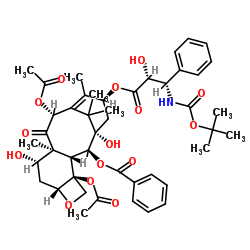
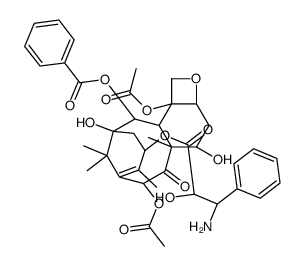
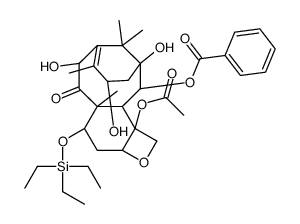
![7,10-Bis[O-(triethylsilyl)]-10-deacetyl Baccatin III structure](https://image.chemsrc.com/caspic/360/149107-84-6.png)