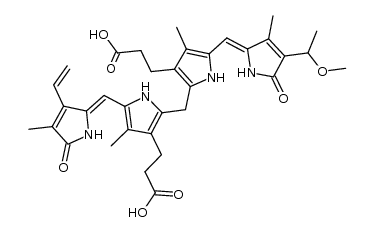
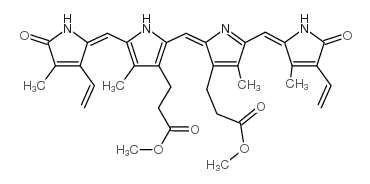
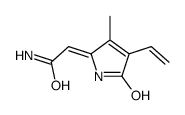
635-65-4
| Name | bilirubin |
|---|---|
| Synonyms |
(4Z,15Z)-Bilirubin IXa
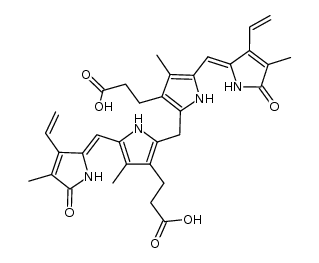
Bilirubin: 21H-Biline-8,12-dipropanoicacid,2,17-diethenyl-1,10,19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-, Biliyubin filirubin Cholerythrin EINECS 211-239-7 (Z,Z)-Bilirubin 3-{2-({3-(2-Carboxyethyl)-4-methyl-5-[(Z)-(3-methyl-5-oxo-4-vinyl-1,5-dihydro-2H-pyrrol-2-ylidene)methyl]-1H-pyrrol-2-yl}methyl)-4-methyl-5-[(Z)-(4-methyl-5-oxo-3-vinyl-1,5-dihydro-2H-pyrrol-2-ylidene)methyl]-1H-pyrrol-3-yl}propanoic acid 1,10,19,22,23,24-hexahydro-2,7,13,17-tetramethyl-1,19-dioxo-3,18-divinyl-Biline-8,12-dipropionic acid bilirubin 1,3,6,7-Tetramethyl-4,5-dicarboxyethyl-2,8-divinyl-(b-13)-dihydrobilenone hemetoidin (Z,Z)-Bilirubin IXa Bilirubin IXa MFCD00005499 |
| Description | Bilirubin is a yellow breakdown product of heme catabolism. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Unconjugated Bilirubin inhibits the cleavage of F485-rVWF73-H, D633-rVWF73-H, and GST-rVWF71-11K by ADAMTS13 in a concentration-dependent manner with a half-maximal inhibitory concentration (IC50) of ~13 μM, ~70 μM, and ~17 μM, respectively. Unconjugated Bilirubin also dose-dependently inhibits the cleavage of multimeric VWF by ADAMTS13 under denaturing conditions[1]. Bilirubin exhibits antioxidant and antimutagenic effects in vitro[2]. |
| References |
| Density | 1.2163 |
|---|---|
| Boiling Point | 641.7°C |
| Melting Point | 192 °C |
| Molecular Formula | C33H36N4O6 |
| Molecular Weight | 584.662 |
| Flash Point | 478.1±37.1 °C |
| Exact Mass | 584.263489 |
| PSA | 164.38000 |
| LogP | 3.15 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.640 |
| Storage condition | −20°C |
| Stability | Stable. Refrigerate. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R62 |
| Safety Phrases | 22-24/25-36-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DU3038000 |
| HS Code | 29339990 |
|
~82% 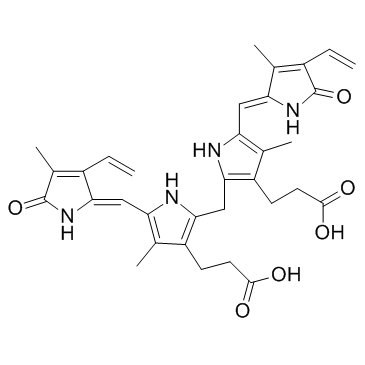
635-65-4 |
| Literature: Monti, Diego; Speranza, Giovanna; Manitto, Paolo Gazzetta Chimica Italiana, 1982 , vol. 112, # 9/10 p. 361 - 366 |
|
~% 
635-65-4 |
| Literature: Monatshefte fur Chemie, , vol. 145, # 3 p. 465 - 482 |
|
~% 
635-65-4 |
| Literature: Gazzetta Chimica Italiana, , vol. 112, # 9/10 p. 361 - 366 |
|
~% 
635-65-4 |
| Literature: Gazzetta Chimica Italiana, , vol. 112, # 9/10 p. 361 - 366 |
|
~% 
635-65-4 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 34, # 3 p. 263 - 274 |
|
~% 
635-65-4 |
| Literature: Journal of Labelled Compounds and Radiopharmaceuticals, , vol. 34, # 3 p. 263 - 274 |
|
~% 
635-65-4 |
| Literature: Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, , vol. 268, p. 197,222 |
|
~% 
635-65-4 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 21, # 4 p. 1005 - 1008 |
| Precursor 8 | |
|---|---|
| DownStream 9 | |
| HS Code | 29339990 |
|---|