154361-50-9
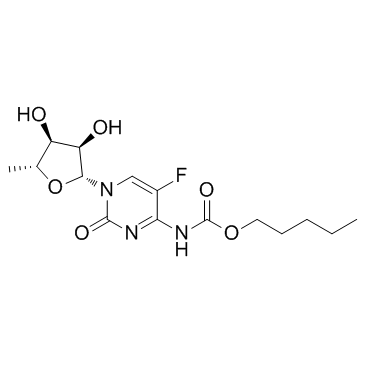
| Name | capecitabine |
|---|---|
| Synonyms |
1-(5-Deoxypentofuranosyl)-5-fluoro-4-{[(pentyloxy)carbonyl]amino}-2(1H)-pyrimidinone
5'-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine Capecitabine 5'-Deoxy-5-fluoro-N4-[(pentyloxy)carbonyl]cytidine Capecytabine Ro 09-1978 Captabin MFCD00930626 XELODA RO-9-1978 Cpecitabine Capecitibine 1-(5-deoxypentofuranosyl)-5-fluoro-4-{[(pentyloxy)carbonyl]amino}pyrimidin-2(1h)-one Ro 09-1978/000 |
| Description | Capecitabine is an oral prodrug that is converted to its active metabolite, fluorouracil (FU), by thymidine phosphorylase. |
|---|---|
| Related Catalog | |
| Target |
DNA/RNA Synthesis[1] |
| In Vitro | Capecitabine is an anti-cancer chemotherapy drug. It is classified as an antimetabolite. Capecitabine is converted into 5′-deoxy-5-fluorocytidine (5′DFCR), 5′-deoxy-5-fluorouridine (5′DFUR) and 5-fluorouracil (5-FU) by carboxylesterases (CES1 and 2), cytidine deaminase (CDD), and thymidine phosphorylase (TP), in both liver and tumour. Capecitabine induces a significant cytotoxic effect in vitro only at high concentrations. Mean IC50 values vary from 860 μM in COLO205 cells to 6000 μM in HCT8 cells[2]. |
| In Vivo | A pharmacokinetic/pharmacodynamic study is carried out in mice bearing HCT 116 xenografts receiving 0.52 and 2.1 mmol/kg/d of Capecitabine by oral gavage. Capecitabine administered at 0.52 mmol/kg/day induces partial control of HCT 116 xenografts tumour growth: growth rate =7.5±0.5 on day 21. Capecitabine 2.1 mmol/kg/day achieves complete control of tumor growth during the treatment period: growth rate =1±0.2 on day 21[2]. |
| Cell Assay | HCT 116, HCT8, HCT15, HT29, SW620 and COLO205 human colon cancer cells are used. Cells are plated on day 1 in 96-well plates at a density of 2500 cells/well for HCT 116, 3500 cells/well for HCT8 and HT29, 5000 cells/well for HCT15, 6000 cells/well for SW620 and 7000 cells/wells for COLO205 in a volume of 150 μL/well. All cell lines are treated on day 2 with increasing concentrations of Capecitabine (0.1-10 mM), 5′DFCR (10 nM-100 μM), 5′DFUR (2.5-500 μM) or 5-FU (0.5-250 μM) for 24 h. After drug exposure, cells are washed once with cold PBS and placed in 200 μL of drug-free medium for 72 h after the end of drug exposure. The cells are then fixed with trichloroacetic acid and stained with sulforhodamine B. Optical densities are measured at 540 nm with a Biohit BP-800. The results are based on three independent experiments performed in triplicate[2]. |
| Animal Admin | Mice[2] Six-week-old C57/Bl6 Nu/Nu mice are used. Bilateral HCT 116 xenografts are obtained by subcutaneous injection of 107 cells/flank. Animals bearing HCT 116 xenografts are treated with vehicle or Capecitabine 0.52 or 2.1 mmol/kg (563 and 2250 mg/m2, respectively) given once daily for 5 consecutive days/week by oral gavage for 3 weeks (days 0-4, 7-11, 14-18). Animals are culled on day 0 at 15, 30 min, 1, 2, 4, 8 and 24 h, and prior to planned treatment on days 7 and 14 after the start of treatment. Three animals per time-point are analysed. At the time of collection, blood is collected in heparin, and plasma isolated and stored at −80°C. The liver is removed immediately and stored in RNAlater solution. Tumours are macro-dissected to remove fibrotic tissue and blood vessels and snap-frozen in liquid nitrogen. |
| References |
[1]. PharmD CM, et al. Capecitabine: A review. Clinical Therapeutics. 2005 Jan; 27(1): 23-44. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 517.6±60.0 °C at 760 mmHg |
| Melting Point | 110-121°C |
| Molecular Formula | C15H22FN3O6 |
| Molecular Weight | 359.350 |
| Flash Point | 266.8±32.9 °C |
| Exact Mass | 359.149261 |
| PSA | 122.91000 |
| LogP | 0.97 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H341-H350-H360FD |
| Precautionary Statements | P201-P308 + P313 |
| Hazard Codes | T |
| Risk Phrases | 45-60-61-68 |
| Safety Phrases | 53-22-36/37-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
![pentyl (5-fluoro-1-((3aR,4R,6R,6aR)-6-methyl-2-oxidotetrahydrofuro[3,4-d][1,3,2]dioxathiol-4-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate structure](https://image.chemsrc.com/caspic/162/1309454-52-1.png)
![5'-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine 2',3'-diacetate structure](https://image.chemsrc.com/caspic/201/162204-20-8.png)