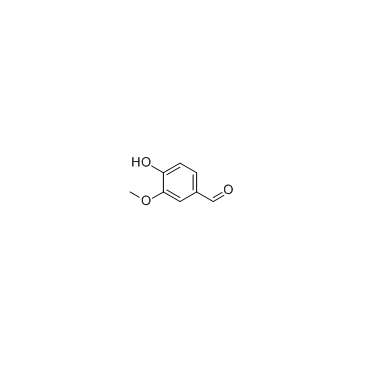
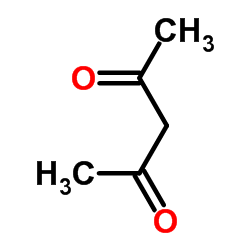
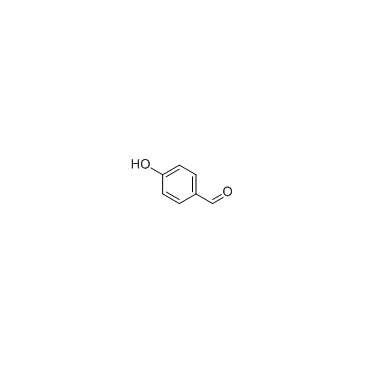
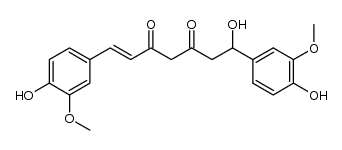
458-37-7
| Name | curcumin |
|---|---|
| Synonyms |
Diferulylmethane
Turmeric yellow curcuma EINECS 207-280-5 diferuloylmethane Natural Yellow 3 gelbwurz (1E,4Z,6E)-5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one (1E,4Z,6E)-5-Hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one curouma halad souchet kurkumin haidr Curcumin (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione MFCD01868798 |
| Description | Curcumin is a natural phenolic compound with diverse pharmacologic effects including anti-inflammatory, antioxidant, antiproliferative and antiangiogenic activities. Curcumin is an inhibitor of p300 histone acetylatransferase ((HATs)) and also shows inhibitory effects on NF-κB and MAPKs. |
|---|---|
| Related Catalog | |
| Target |
Keap1-Nrf2[1], Histone acetyltransferase[6] |
| In Vitro | Curcumin exerts its chemopreventive effects partly through the activation of nuclear factor (erythroid-2 related) factor 2 (Nrf2) and its antioxidant and phase II detoxifying enzymes[1]. Curcumin inhibits T47D cells growth, with IC50s of 25, 19 and 17.5 μM for 24, 48 and 72 h MTT assays respectively. IC50s of curcumin and silibinin mixture against T47D cells, are 17.5, 15, and 12 μM for 24, 48, and 72 h exposure times, respectively[2]. Curcumin (2.5-80 μM) induces apoptotic cell death in AGS and HT-29 cell lines, and the IC50 is 21.9±0.1, 40.7±0.5 μM, respectively, in both AGS and HT-29 cell lines. Curcumin-induced apoptosis requires caspase activities in AGS and HT-29 cells. Curcumin induces ER Ca2+ decline and mitochondrial Ca2+ overloading[3]. Curcumin induces the G2/M cell cycle arrest of LNCaP and PC-3 cells in a dose dependent manner. Curcumin upregulates the protein level of NF-kappaB inhibitor IkappaBalpha and downregulates protein levels of c-Jun and AR[5]. |
| In Vivo | Curcumin (10 mg/kg, p.o.) significantly prevents decrease in the percentage of sucrose consumption, as compared to the CMS-exposed rats. Curcumin treatment results in significant prevention of increase in TNF-α and IL-6 levels in stressed rats[4]. Curcumin decreases binding of p300/CREB-binding protein (CBP) at the brain-derived neurotrophic factor (BDNF) promoter at 20 mg/kg (i.p.), reduces binding of P300/CBP at the BDNF promoter at 40 mg/kg, and decreases binding all the four proteins of p300/CBP and H3K9ac/H4K5ac at the BDNF promoter at 60 mg/kg in chronic constriction injury (CCI) rats[6]. |
| Cell Assay | T47D breast cancer cell line is grown in RPMI 1640 supplemented with 10% FBS, 2 mg/mL sodium bicarbonate, 0.05 mg/mL penicillin G, 0.08 mg/mL streptomycin. Culture is maintained on plastic flask and incubated at 37°C in 5% CO2. After growing sufficient amount of cells, cytotoxic effect of silibinin and curcumin is studied by 24, 48 and 72 h MTT assays in which 1000 cell/well are cultivated in a 96 well plate. After 24 h incubation in 37°C with humidified atmosphere containing 5% CO2, the cells are treated with serial concentrations of curcumin (5, 10, 20, 30, 40, 50, 60, 80, 100 µM), silibinin (20, 40, 60, 80, 100, 120, 140, 180, 200 µM), and curcumin-silibinin mixture (each of them 5, 10, 20, 30, 40, 50, 60, 80, 100 µM) for 24, 48 and 72 h in the quadruplicate manner, in addition to cells with 200 μL culture medium containing 10% DMSO for control. After incubation, the medium of all wells of the plate are exchanged with fresh medium and the cells are leaved for 24 h in incubator. Then, medium of all wells are removed carefully and 50 μL of 2 mg/mL MTT dissolved in PBS is added to each wells and the plate is covered with aluminum foil and incubated for 4.5 h again. After removing content of the wells, 200 μL pure DMSO is added to the wells. Then, 25 μL Sorensen’s glycine buffer is added and immediately absorbance of each wells is read in 570 nm using EL×800 Microplate Absorbance Reader with reference wavelength of 630 nm. |
| Animal Admin | Curcumin (10 mg/kg), freshly suspended in saline, is administrated by oral gavage once a day for 3 weeks. Forty rats are randomLy assigned to 4 groups (n=10/each group): group I receives saline and serves as control, group II receives curcumin, group III is exposed to CMS andreceive saline and group IV are subjected to CMS andreceive curcumin. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 593.2±50.0 °C at 760 mmHg |
| Melting Point | 183 °C |
| Molecular Formula | C21H20O6 |
| Molecular Weight | 368.380 |
| Flash Point | 209.7±23.6 °C |
| Exact Mass | 368.125977 |
| PSA | 96.22000 |
| LogP | 2.85 |
| Vapour density | 13 (vs air) |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.672 |
| Storage condition | −20°C |
| Water Solubility | Slightly soluble (hot) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | MI5230000 |
| HS Code | 2932999099 |
|
~91% 
458-37-7 |
| Literature: Journal of Agricultural and Food Chemistry, , vol. 57, # 22 p. 11041 - 11046 |
|
~% 
458-37-7 |
| Literature: US2006/160812 A1, ; Page/Page column 2 ; |
|
~% 
458-37-7
Detail
|
| Literature: Journal of Natural Products, , vol. 61, # 12 p. 1531 - 1534 |
|
~% 
458-37-7
Detail
|
| Literature: Journal of Natural Products, , vol. 61, # 12 p. 1531 - 1534 |
|
~% 
458-37-7
Detail
|
| Literature: Journal of Natural Products, , vol. 61, # 12 p. 1531 - 1534 |
|
~7% 
458-37-7 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 24, # 2 p. 685 - 690 |
|
~% 
458-37-7 |
| Literature: Medicinal Chemistry Research, , vol. 20, # 4 p. 503 - 510 |
|
~% 
458-37-7 |
| Literature: Chemical & Pharmaceutical Bulletin, , vol. 35, # 8 p. 3298 - 3304 |
| Precursor 5 | |
|---|---|
| DownStream 8 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |