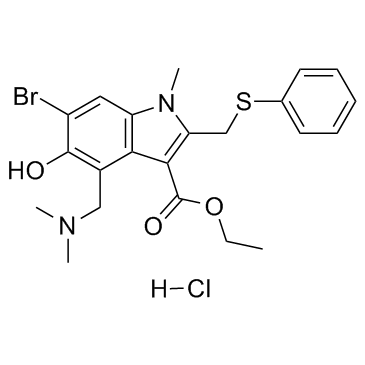
131707-23-8
| Name | ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-(phenylsulfanylmethyl)indole-3-carboxylate,hydrochloride |
|---|---|
| Synonyms |
1-methyl-2-phenylthiomethyl-3-carbethoxy-4-dimetylaminomethyl-5-hydroxy-6-bromoindole hydrogen chloride
Arbidol Hydrochloride 6-Bromo-4-((dimethylamino)methyl)-5-hydroxy-1-methyl-2-((phenylthio)methyl)-1H-indole-3-carboxylic Acid Ethyl Ester Moonohydrochloride Ethyl 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylsulfanyl)methyl]-1H-indole-3-carboxylate hydrochloride (1:1) 1-methyl-2-phenylthiomethyl-3-carbethoxy-4-dimethylaminomethyl-5-oxy-6-bromindol hydrochloride Arbidol Hydrochloride Hydrate arbidol hydrogen chloride 1H-Indole-3-carboxylic acid, 6-bromo-4-[(dimethylamino)methyl]-5-hydroxy-1-methyl-2-[(phenylthio)methyl]-, ethyl ester, hydrochloride (1:1) umifenovir hydrogen chloride Arbidol HCl MFCD00333871 Arbidol umifenovir hydrochloride Arbidol (hydrochloride) |
| Description | Arbidol (Umifenovir) hydrochloride is an broad-spectrum antiviral chemical agent which can inhibit cell entry of enveloped viruses by blocking viral fusion with host cell membraneIC50 value:Target: Antiviral; Anti-influenza agentin vitro: Arbidol was found to present potent inhibitory activity against enveloped and non-enveloped RNA viruses, including FLU-A, RSV, HRV 14 and CVB3 when added before, during, or after viral infection, with 50% inhibitory concentration (IC50) ranging from 2.7 to 13.8 microg/ml.However, arbidol showed selective antiviral activity against AdV-7, a DNA virus, only when added after infection (therapeutic index (TI) = 5.5) [1]. Arb interacts with the polar head-group of phospholipid at the membrane interface. Fluorescence studies of interactions between Arb and either tryptophan derivatives or membrane peptides reconstituted into liposomes show that Arb interacts with tryptophan in the micromolar range. Interestingly, apparent binding affinities between lipids and tryptophan residues are comparable with those of Arb IC50 of the hepatitis C virus (HCV) membrane fusion [2]. Arbidol not only prevented the cytopathic effect (CPE) of CVB(5), as demonstrated in an MTT colorimetric assay, when added during or after viral infection, with a 50% inhibitory concentration (IC(50)) from 2.66 to 6.62 microg/ml, but it also decreased the CVB(5)-RNA level in infected host cells, as shown in semi-quantitative RT-PCR [3].in vivo: Orally administered arbidol at 50 or 100 mg/kg/day beginning 24 h pre-virus exposure for 6 days significantly reduced mean pulmonary virus yields and the rate of mortality in mice infected with FLU-A (A/PR/8/34 H1N1) [1]. BALB/c mice were used as an animal model to test the Arbidol activity in vivo. Orally administered Arbidol at 50 mg/kg body weight/day (once a day) significantly reduced mean virus yields in the lungs and heart as well as mortality after infection for 6 days [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 591.8ºC at 760 mmHg |
|---|---|
| Melting Point | 133-137ºC |
| Molecular Formula | C22H26BrClN2O3S |
| Molecular Weight | 513.875 |
| Flash Point | 311.7℃ |
| Exact Mass | 512.053589 |
| PSA | 80.00000 |
| LogP | 5.97900 |
| Vapour Pressure | 1.34E-14mmHg at 25°C |
| Storage condition | Hygroscopic, Refrigerator, Under Inert Atmosphere |
| Stability | Hygroscopic |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/38 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |