5986-55-0
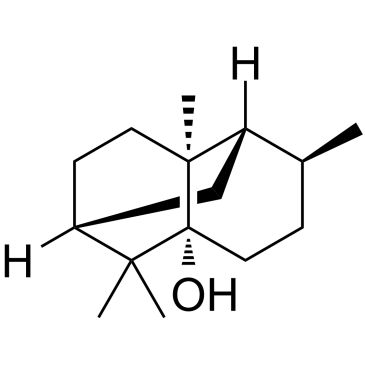
| Name | patchouli alcohol |
|---|---|
| Synonyms |
Patchoulic alcohol
(1R,3R,6S,7S,8S)-2,2,6,8-Tetramethyltricyclo[5.3.1.0]undecan-3-ol (-)-patchouli alcohol patchouli alcohol (1R,4S,4aS,6R,8aS)-4,8a,9,9-tetramethyloctahydro-1,6-methanonaphthalen-1(2H)-ol [1R-(1a,4b,4aa,6b,8aa)]-Octahydro-4,8a,9,9-tetramethyl-1,6-methanonaphthalen-1(2H)-ol patchoulol Patchoulialcohol Patchouli Camphor Patchoulanol 4beta,4aalpha,6beta,8aalpha)]-ph (1R,4S,4aS,6R,8aS)-Octahydro-4,8a,9,9-tetramethyl-1,6-methanonaphthalen-1(2H)-ol EINECS 227-807-2 |
| Description | Patchouli alcohol is a natural tricyclic sesquiterpene extracted from Pogostemon cablin (Blanco) Benth, and exhibits anti-Helicobacter pylori and anti-inflammatory properties[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 287.4±8.0 °C at 760 mmHg |
| Melting Point | 56°; mp (racemate) 39-40° (Danishevsky, Dumas); mp 46-47° (Mirrington, Schmalzl) |
| Molecular Formula | C15H26O |
| Molecular Weight | 222.366 |
| Flash Point | 120.2±10.9 °C |
| Exact Mass | 222.198364 |
| PSA | 20.23000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.515 |
|
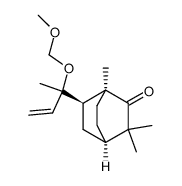
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
|
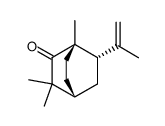
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
|
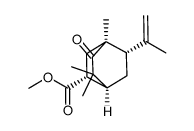
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
|
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
|
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
|
~% 
5986-55-0 |
| Literature: Tetrahedron Asymmetry, , vol. 16, # 24 p. 3992 - 3997 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |