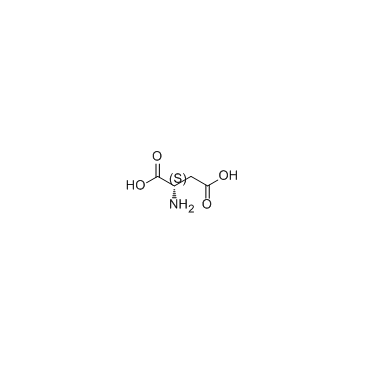
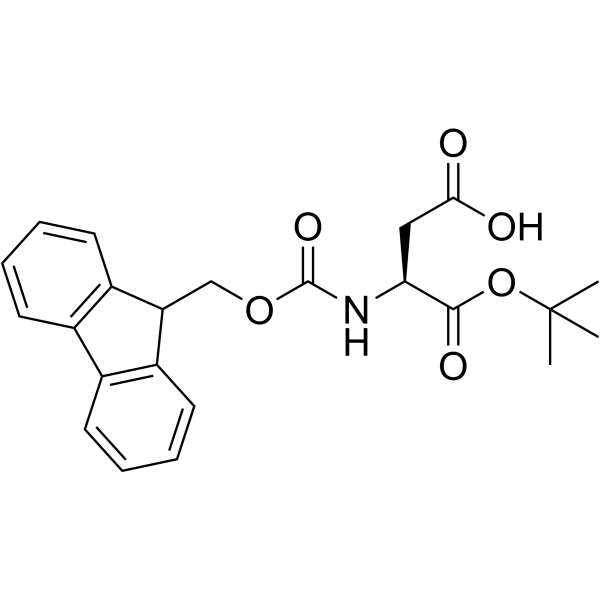
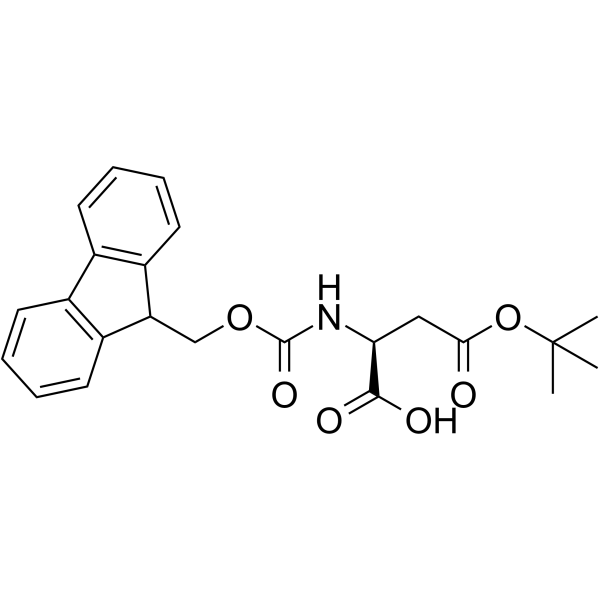
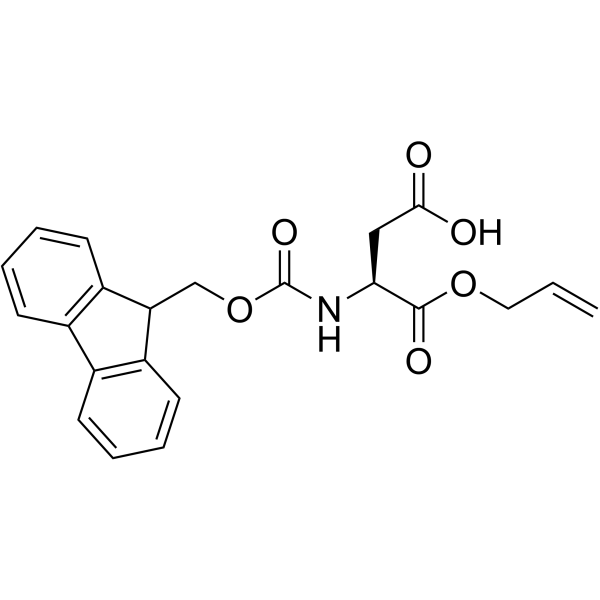
136083-57-3
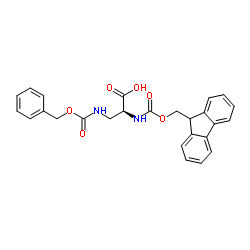
| Name | (2R)-2-(9H-fluoren-9-ylmethoxycarbonylamino)butanedioic acid |
|---|---|
| Synonyms |
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-D-aspartic acid
N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-aspartic acid N-Fmoc-L-aspartic acid N-(9-fluorenylmethoxycarbonyl)-L-aspartic acid Fmoc-D-aspartic acid Fmoc-D-Asp-OH (2S)-2-{[(9H-Fluoren-9-ylmethoxy)carbonyl]amino}succinic acid N-Fmoc-D-aspartic Acid Fmoc-aspartic acid MFCD01318740 |
| Description | Fmoc-D-Asp-OH is an aspartic acid derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 587.2±45.0 °C at 760 mmHg |
| Molecular Formula | C19H17NO6 |
| Molecular Weight | 355.341 |
| Flash Point | 308.9±28.7 °C |
| Exact Mass | 355.105591 |
| PSA | 112.93000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.628 |
| Storage condition | Store at RT. |
| Hazard Codes | Xn-F,Xn |
|---|---|
| Risk Phrases | R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . R11:Highly Flammable. R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S22-S24/25-S36/37/39-S26-S16 |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~91% 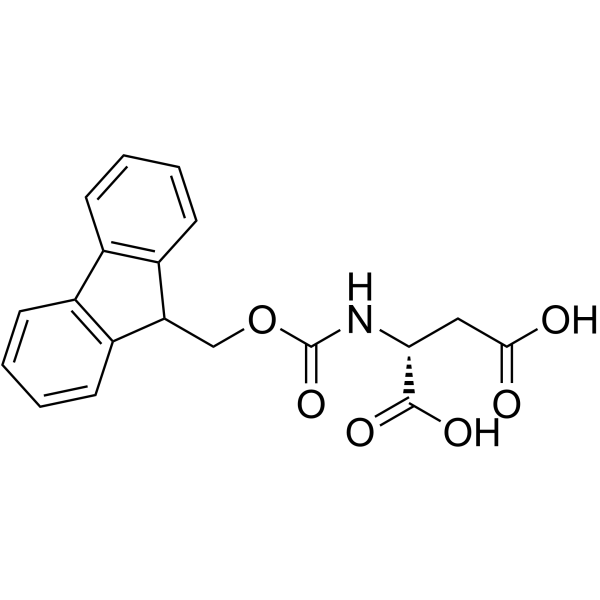
136083-57-3 |
| Literature: Commissariat A L'Energie Atomique; Universite De Picardie Jules Verne Patent: US2007/142324 A1, 2007 ; Location in patent: Page/Page column 8 ; |
|
~96% 
136083-57-3 |
| Literature: Mehta, Anita; Jaouhari, Rabih; Benson, Timothy J.; Douglas, Kenneth T. Tetrahedron Letters, 1992 , vol. 33, # 37 p. 5441 - 5444 |
|
~% 
136083-57-3 |
| Literature: Journal of Peptide Science, , vol. 16, # 3 p. 159 - 164 |
|
~35% 
136083-57-3 |
| Literature: Chinchilla, Rafael; Dodsworth, David; Najera, Carmen; Soriano, Jose Tetrahedron Letters, 2001 , vol. 42, # 43 p. 7579 - 7581 |
|
~75% 
136083-57-3 |
| Literature: Sakina; Kawazura; Morihara; Yajima Chemical and Pharmaceutical Bulletin, 1988 , vol. 36, # 10 p. 3915 - 3919 |
|
~% 
136083-57-3 |
| Literature: Journal of Photochemistry and Photobiology A: Chemistry, , vol. 241, p. 52 - 57 |
|
~% 
136083-57-3 |
| Literature: Journal of Photochemistry and Photobiology A: Chemistry, , vol. 241, p. 52 - 57 |
|
~84% 
136083-57-3 |
| Literature: Singh, Suneel P.; Michaelides, Alex; Merrill, A. Rod; Schwan, Adrian L. Journal of Organic Chemistry, 2011 , vol. 76, # 16 p. 6825 - 6831 |
| Precursor 6 | |
|---|---|
| DownStream 6 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |