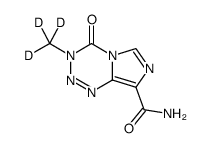
208107-14-6
| Name | 4-oxo-3-(trideuteriomethyl)imidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide |
|---|---|
| Synonyms |
Temodar-d3
Methazolastone-d3 Temozolomide-d3 Temodal-d3 |
| Description | Temozolomide-d3 (NSC 362856-d3) is the deuterium labeled Temozolomide. Temozolomide (NSC 362856) is an oral active DNA alkylating agent that crosses the blood-brain barrier. Temozolomide is also a proautophagic and proapoptotic agent. Temozolomide is effective against tumor cells that are characterized by low levels of O6-alkylguanine DNA alkyltransferase (OGAT) and a functional mismatch repair system. Temozolomide has antitumor and antiangiogenic effects[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 2.004g/cm3 |
|---|---|
| Boiling Point | 526.592ºC at 760 mmHg |
| Melting Point | 202-204ºC |
| Molecular Formula | C6H3D3N6O2 |
| Molecular Weight | 197.16900 |
| Flash Point | 272.273ºC |
| Exact Mass | 197.07400 |
| PSA | 108.17000 |
| Vapour Pressure | 0mmHg at 25°C |
| Index of Refraction | 1.895 |