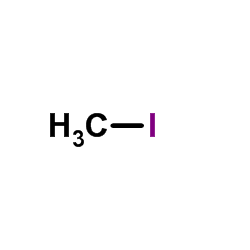
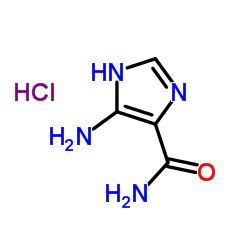
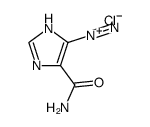
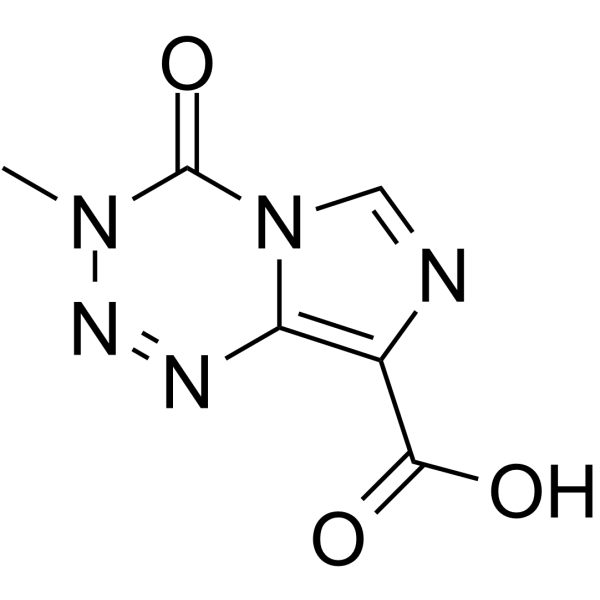
85622-93-1
| Name | temozolomide |
|---|---|
| Synonyms |
Temodal
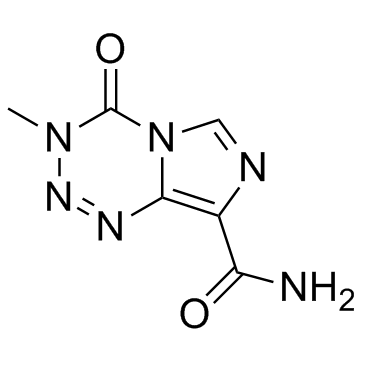
Temozolamide 3-Methyl-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide TZM M&B 39831 TEMODAR Temozolomide MFCD00866492 temosolomide |
| Description | Temozolomide (NSC 362856; CCRG 81045) is an oral DNA alkylating agent used to treat some brain cancers. |
|---|---|
| Related Catalog | |
| Target |
DNA alkylator[1] |
| In Vitro | Temozolomide (TZM) is a methylating agent that crosses the blood-brain barrier and is indicated for malignant gliomas and metastatic melanomas. Temozolomide is effective against tumor cells that are characterized by low levels of O6-alkylguanine DNA alkyltransferase (OGAT) and a functional mismatch repair system (MR)[1]. Determination of the IC50 for Temozolomide (TZM) in different cell lines gave values ranging from 14.1 to 234.6 μM that fell into two clearly differentiated groups: cell lines with low IC50 values (<50 μM), which include A172 (14.1±1.1 μM) and LN229 cells (14.5±1.1 μM), and those with high IC50 values (>100 μM), which include SF268 (147.2±2.1 μM) and SK-N-SH cells (234.6±2.3 μM)[2]. |
| In Vivo | Temozolomide (TZM), as a single agent, does not significantly increase mdian survival time (MST) with respect to control. Noteworthy, intracranial injection of NU1025, immediately before the administration of 100 or 200 mg/kg Temozolomide, significantly increases lifespans with respect to controls or to groups treated with Temozolomide only. When Temozolomide is fractionated, the increase in lifespan (ILS) obtained with this schedule is higher than that observed when NU1025 is combined with a single injection of Temozolomide (statistical comparison of survival curves: NU1025 intracranially+Temozolomide 100 mg/kg×2 vs NU1025+Temozolomide 200 mg/kg; P=0.023)[1]. |
| Cell Assay | The murine lymphoma cell line L5178Y of DBA/2 (H-2d/H-2d) origin is cultured in RPMI-1640 containing 10% fetal calf serum and antibiotics. Inhibition of PARP is obtained by treating cells (105 cells/mL) with 8-hydroxy-2-methylquinazolin-4[3H]-1 (NU1025), at a concentration (25 μM) that abrogates PARP activity. Cells are then exposed to Temozolomide (7.5-125 μM) and are cultured for 3 days. Cell growth is evaluated by counting viable cells in quadruplicate, and apoptosis is assessed by flow cytometry analysis of DNA content. Long-term survival is analyzed by colony-formation assay[1]. |
| Animal Admin | Mice[1] Male B6D2F1 (C57BL/6×DBA/2) mice are anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) in 0.9% NaCl solution (10 mL/kg intraperitoneally). L5178Y cells (104 in 0.03 mL RPMI-1640) are then injected intracranially, through the center-middle area of the frontal bone to a 2-mm depth, using a 0.1-mL glass microsyringe and a 27-gauge disposable needle. To evaluate tumor cell growth, brains are fixed in 10% phosphate-buffered formaldehyde, and histologic sections (5 μm) are cut along the axial plane, stained with hematoxylin-eosin, and analyzed by light microscopy. Temozolomide is dissolved in DMSO (40 mg/mL), diluted in saline (5 mg/mL), and administered intraperitoneally on day 2 after tumor injection at 100 mg/kg or 200 mg/kg, doses commonly used for in vivo preclinical studies. Because cytotoxicity induced by Temozolomide and PARP inhibitors can be improved by fractionated modality of treatment, in selected groups a total dose of 200 mg/kg Temozolomide is divided in 2 doses of 100 mg/kg given on days 2 and 3. |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 526.6±42.0 °C at 760 mmHg |
| Melting Point | 212°C dec. |
| Molecular Formula | C6H6N6O2 |
| Molecular Weight | 194.151 |
| Flash Point | 272.3±27.9 °C |
| Exact Mass | 194.055222 |
| PSA | 108.17000 |
| LogP | -1.32 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.895 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H335-H350-H360 |
| Precautionary Statements | P201-P261-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic;Xi: Irritant; |
| Risk Phrases | R45 |
| Safety Phrases | S24/25-S45-S36/37-S26-S53 |
| RIDADR | NONH for all modes of transport |
| RTECS | NJ5927050 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


![3-(hydroxymethyl)-4-oxo-3,4-dihydroimidazo[5,1-d][1,2,3,5]tetrazine-8-carboxamide structure](https://image.chemsrc.com/caspic/162/1161825-88-2.png)