101477-54-7
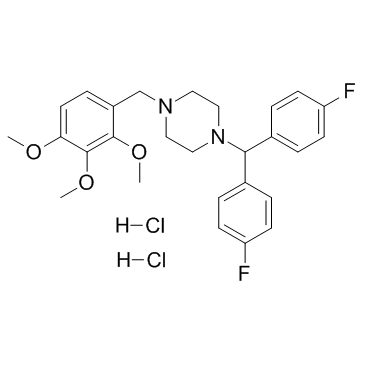
| Name | 1-[Bis(4-fluorophenyl)methyl]-4-(2,3,4-trimethoxybenzyl)piperazine Dihydrochloride |
|---|---|
| Synonyms |
1-[Bis(4-fluorophenyl)methyl]-4-[(2,3,4-trimethoxyphenyl)methyl]piperazine dihydrochloride
Lomerizine HCl 1-[bis(4-fluorophenyl)methyl]-4-[(2,3,4-trimethoxyphenyl)methyl]piperazine,dihydrochloride MFCD01703867 Lomerizine Hydrochloride Piperazine, 1-[bis(4-fluorophenyl)methyl]-4-[(2,3,4-trimethoxyphenyl)methyl]-, hydrochloride (1:2) Lomerizine dihydrochloride 1-(bis(4-fluorophenyl)methyl)-4-((2,3,4-trimethoxyphenyl)methyl)piperazine dihydrochloride 1-[Bis(4-fluorophenyl)methyl]-4-(2,3,4-trimethoxybenzyl)piperazine dihydrochloride |
| Description | Lomerizine dihydrochloride is an antagonist of L- and T-type voltagegated calcium channels. |
|---|---|
| Related Catalog | |
| Target |
T-type calcium channel L-type calcium channel |
| In Vitro | Lomerizine is an antagonist of L- and T-type voltagegated calcium channels and transient receptor potential channel 5 transient receptor potential channels. Lomerizine is a dual L/T-type channel blocker used for prophylaxis of migraine. To demonstrate the effectiveness of Lomerizine in limiting intracellular [Ca2+], its ability to inhibit glutamate-induced death of motor neurons and the associated rise in cytosolic [Ca2+] is evaluated. Lomerizine inhibits the low- and high-voltage activated Ca2+ currents in dissociated rat brain neurons at a threshold concentration of 0.01 μM and IC50 of 1.9 μM and H2O2-induced Ca2+ influx in hippocampal neurons is inhibited by 1 μM Lomerizine. Pre-treatment with 1 μM Lomerizine significantly reduces acute death of motor neurons in spinal cord-DRG cultures exposed to 50 μM glutamate, a concentration that kills approximately 40% of motor neurons in the culture by 6 h, and inhibits the rise in cytosolic [Ca2+] that occurs with glutamate treatment. 0.5 μM Lomerizine is sufficient to significantly prevent the mitochondrial fragmentation of mitochondria induced by SOD1G93A[1]. Lomerizine increases the cytotoxicity of Adriamycin (ADM) and the apoptosis induced by ADM or Vincristine (VCR) in K562/ADM cells. At the concentration of 3, 10 and 30μM, Lomerizine reduces the IC50 value of ADM from 79.03 μM to 28.14, 8.16 and 3.16 μM, respectively. Lomerizine increases the intracellular accumulation of ADM and inhibits the efflux of Rh123 in K562/ ADM cells. No change in P-gp expression is observed after the treatment of Lomerizine for 72 h. Lomerizine has strong reversal effect on MDR in K562/ADM cells by inhibiting P-gp function[2]. |
| In Vivo | To determine whether Ca2+ signaling molecules mediate NMDA-induced neurotoxicity in p50-deficient mice, the neuroprotective effects of chemical reagents are examined, which act on the Ca2+-signaling pathway including CaN activation, on NMDA-induced RGC death. The p50-deficient mice at 2 months of age, showing normal RGC survival, undergo intraperitoneal pretreatments with a NMDA antagonist, MK801 or Memantine; calcium blocker, Lomerizine; and CaN inhibitor, Tacrolimus, daily for 1 week before the injection of 5 nM NMDA. The chronic administration of Lomerizine or Tacrolimus to KO mice for 6 months results in an increase in surviving RGC numbers (p<0.0001)[3]. Lomerizine (KB-2796; 0.3 and 1 mg/kg, i.v.) dose-dependently increases cerebral blood flow significantly at 30 min and 15 min, respectively, after its administration. Lomerizine (1 mg/kg, i.v.) significantly attenuates the expression of c-Fos-like immunoreactivity in the ipsilateral frontoparietal cortex[4]. |
| Cell Assay | MTT assay is used to determine the influence of Lomerizine on the cytotoxicity of Adriamycin (ADM). The effect of Lomerizine (3, 10 and 30 μM) on the apoptosis induced by ADM and Vincristine (VCR) in K562/ADM cells is detected using flow cytometry. Intracellular accumulation of ADM is measured by fluorescence spectrophotometry. Flow cytometry is used to investigate the efflux of Rhodamine 123 (Rh123) and the expression of P-glycoprotein (P-gp) in K562/ADM cells[2]. |
| Animal Admin | Mice[3] The p50-deficient mice and wild-type mice aged 2 months are daily pre-treated intraperitoneally with Memantine (10 mg/kg), MK-801 (0.5 mg/kg), Lomerizine (1 mg/kg), or Tacrolimus (2, 0.5 and 0.2 mg/kg) for one week before the NMDA injection. These mice are given an intravitreous injection of 5 nM NMDA, which is a relatively low concentration for causing neurotoxicity[3]. Rats[4] Male Wistar rats weighing 250 to 350 g are housed in an air-conditioned room at 25±0°C with 55±5% humidity and given food and water ad libitum. Lomerizine is injected i.v. in a volume of 1 mL/kg body weight. Effects of Lomerizine (0.3 mg/kg, i.v., or 1 mg/kg, i.v.) are measured on cerebral cortical blood flow measured by laser Doppler flowmetry (CBFLDF) in anaesthetized rats[4]. |
| References |
| Boiling Point | 527.3ºC at760mmHg |
|---|---|
| Melting Point | 214-218ºC |
| Molecular Formula | C27H32Cl2F2N2O3 |
| Molecular Weight | 541.457 |
| Flash Point | 272.7ºC |
| Exact Mass | 540.175781 |
| PSA | 34.17000 |
| LogP | 6.37760 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H410 |
| Precautionary Statements | P273-P501 |
| Hazard Codes | Xn,N |
| Risk Phrases | 22-50 |
| Safety Phrases | 61 |
| RIDADR | UN 3077 9 / PGIII |
| RTECS | TK9189000 |
|
~% 
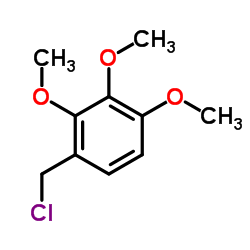
101477-54-7 |
| Literature: US4663325 A1, ; |
|
~38% 
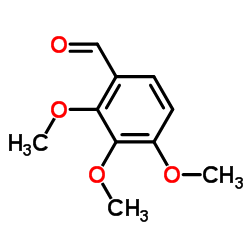
101477-54-7 |
| Literature: Ohtaka; Kanazawa; Ito; Tsukamoto Chemical and Pharmaceutical Bulletin, 1987 , vol. 35, # 8 p. 3270 - 3275 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |