38090-53-8
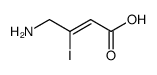
| Name | trans-4-Aminocrotonicacid |
|---|---|
| Synonyms |
MFCD00673818
4-Amino-trans-crotonsaeure 4-amino-trans-crotonic acid trans-4-Aminocrotonic acid,TACA 4-amino-2-butenoic acid trans-4-aminocrotonic acid |
| Description | TACA (trans-4-Aminocrotonic acid) is a potent agonist of GABAA and GABAC receptors (KD= 0.6 μM). TACA also is GABA uptake inhibitor and substrate for GABA-T. TACA produces late biphasic responses in the MPG neurons[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
KD: 0.6 μM (GABAC)[1]. |
| In Vivo | TACA is a potent competitive inhibitor of GABA uptake in rat brain slices and thus is possibly a substrate for the GABA uptake system[3]. |
| References |
| Density | 1.169 g/cm3 |
|---|---|
| Boiling Point | 300.4ºC at 760 mmHg |
| Melting Point | >158°C (lit.) |
| Molecular Formula | C4H7NO2 |
| Molecular Weight | 101.10400 |
| Flash Point | 135.5ºC |
| Exact Mass | 101.04800 |
| PSA | 63.32000 |
| LogP | 0.28620 |
| Safety Phrases | S22-S24/25 |
|---|
|
~% 
38090-53-8 |
| Literature: Musashi Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, 1954 , vol. 297, p. 71 Full Text View citing articles Show Details Johnston et al. J.Neurochem., 1975 , vol. 24, p. 157 |
|
~% 
38090-53-8 |
| Literature: Merck and Co., Inc. Patent: US4579851 A1, 1986 ; US 4579851 A |
|
~% 
38090-53-8 |
| Literature: Liu, Siming; Hanzlik, Robert P. Journal of Medicinal Chemistry, 1992 , vol. 35, # 6 p. 1067 - 1075 |
|
~53% 
38090-53-8 |
| Literature: Allan; Johnston; Twitchin Australian Journal of Chemistry, 1980 , vol. 33, # 5 p. 1115 - 1122 |
|
~% 
38090-53-8 |
| Literature: Balenovic et al. Journal of Organic Chemistry, 1954 , vol. 19, p. 1589,1591 |
|
~% 
38090-53-8 |
| Literature: Allan,R.D.; Twitchin,B. Australian Journal of Chemistry, 1978 , vol. 31, p. 2283 - 2289 |
|
~% 
38090-53-8 |
| Literature: Rambaud Bulletin de la Societe Chimique de France, 1936 , vol. <5>3, p. 139 |
| Precursor 7 | |
|---|---|
| DownStream 1 | |