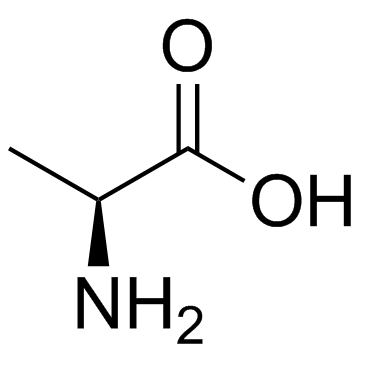
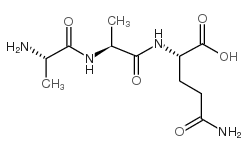
39537-23-0
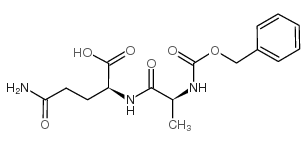
| Name | (2S)-5-amino-2-[[(2S)-2-aminopropanoyl]amino]-5-oxopentanoic acid |
|---|---|
| Synonyms |
UNII-U5JDO2770Z
(2S)-2-((2S)-2-Aminopropanoylamino)-4-carbamoylbutanoic acid ZY1&VMYVQ2VZ &&L-L Form glutaminic acid Gln L-Ala-L-Gln EINECS 475-290-9 glutamine L-Gln L-Alanyl-L-glutamine Levoglutamide (S)-5-Amino-2-[(S)-2-aminopropanamido]-5-oxopentanoic acid L-Glutamine,L-alanyl (2S)-2-amino-4-carbamoylbutanoic acid Alanyl-glutamine,Glutamine-S Ala-Gln (S)-(+)-Glutamine 5-Hydroxy-5-imino-L-norvaline N(2)-L-alanyl-L-glutamine Glutamine-S Alanyl-glutamine L-Glutamic Acid g-Amide L-Glutamic acid γ-amide N-L-alanyl-L-glutamine L-Glutamine l-alanyl-l-glutamin l-(+)-glutamic acid-5-amide L-Glutamic acid 5-amide H-Ala-Gln-OH (2S)-5-Amino-2-{[(2S)-2-aminopropanoyl]amino}-5-oxopentanoic acid MFCD00133046 S(+)-Glutamic acid 5-amide Alanyl glutamine |
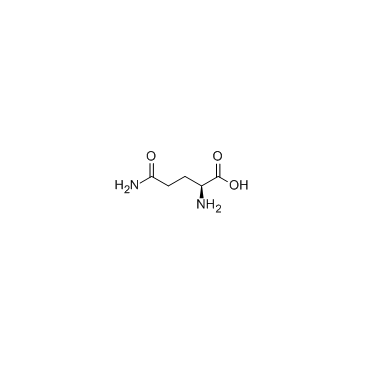
| Description | L-Alanyl-L-glutamine, a glutamine dipeptide, is benefit for the antioxidant system, attenuating inflammation, and may modulate the heat shock protein (HSP) response in catabolic situations[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | L-Alanyl-L-glutamine (2 mM; 24 hours) partially or fully attenuates chronic insulin secretion, and IR-β and COX IV levels which are decreased when β-cells were exposed to macrophages following stimulation in vitro (IMMs)[1]. L-Alanyl-L-glutamine -dependent β-cell protection mediated by coordinated effects on the glutamine-GSH axis, and the HSP pathway, maintenance of mitochondrial metabolism and stimulus-secretion coupling essential for insulin release[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 615℃ |
| Melting Point | 215 °C |
| Molecular Formula | C8H15N3O4 |
| Molecular Weight | 217.22 |
| Flash Point | >110° |
| Exact Mass | 146.069138 |
| PSA | 106.41000 |
| LogP | -1.28 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.564 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S23-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| HS Code | 2924199090 |
|
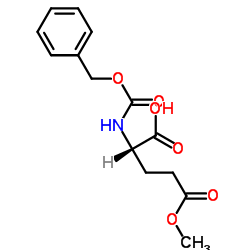
~64% 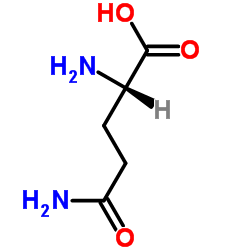
39537-23-0 |
| Literature: Zhao, Yufen; Tang, Guo; Zhou, Ning; Hu, Liming; Chen, Yong Patent: US2005/233977 A1, 2005 ; Location in patent: Page/Page column 4-5 ; |
|
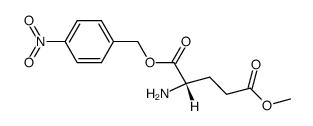
~50% 
39537-23-0 |
| Literature: Zhao, Yufen; Tang, Guo; Zhou, Ning; Hu, Liming; Chen, Yong Patent: US2005/233977 A1, 2005 ; Location in patent: Page/Page column 4 ; |
|
~% 
39537-23-0 |
| Literature: Otsuka Pharmaceutical Co., Ltd. Patent: US4474754 A1, 1984 ; |
|
~% 
39537-23-0 |
| Literature: Shimonishi,Y. Bulletin of the Chemical Society of Japan, 1964 , vol. 37, p. 200 - 203 |
|
~% 
39537-23-0 |
| Literature: Shimonishi,Y. Bulletin of the Chemical Society of Japan, 1964 , vol. 37, p. 200 - 203 |
|
~% 
39537-23-0 |
| Literature: Shimonishi,Y. Bulletin of the Chemical Society of Japan, 1964 , vol. 37, p. 200 - 203 |
|
~% 
39537-23-0 |
| Literature: Shimonishi,Y. Bulletin of the Chemical Society of Japan, 1964 , vol. 37, p. 200 - 203 |
|
~% 
39537-23-0 |
| Literature: Shimonishi,Y. Bulletin of the Chemical Society of Japan, 1964 , vol. 37, p. 200 - 203 |
| Precursor 5 | |
|---|---|
| DownStream 3 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |