313368-91-1
| Name | Lumateperone |
|---|---|
| Synonyms |
Lumateperone
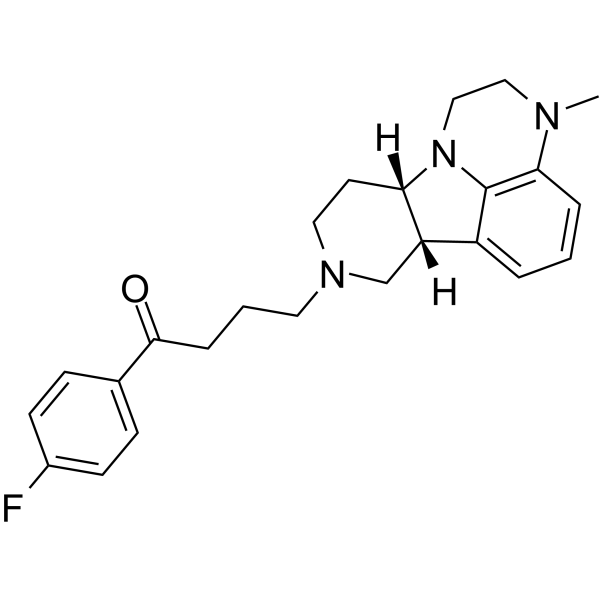
1-(4-Fluorophenyl)-4-[(6bR,10aS)-3-methyl-2,3,6b,9,10,10a-hexahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]-1-butanone UNII:70BSQ12069 ITI-007 ITI-722 |
| Description | Lumateperone (ITI-007) is a 5-HT2A receptor antagonist (Ki = 0.54 nM), a partial agonist of presynaptic D2 receptors and an antagonist of postsynaptic D2 receptors (Ki = 32 nM), and a dopamine D1 receptor modulator. Lumateperone has anticancer activity and can also be used in studies of psychiatric disorders such as schizophrenia[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Lumateperone (2-30 μM) has anti-tumor activity and can inhibit cell proliferation in a dose-dependent manner[1]. Cell Proliferation Assay[1] Cell Line: RPMI-8226 cells Concentration: 2-30 μM Incubation Time: 48 hours Result: Inhibited cell growth with the IC50 value of 17.30 μM. |
| In Vivo | Lumateperone (i.p., 1-10 mg/kg) promotes NMDA and AMPA-induced currents in a dopamine D1 receptor-dependent manner and increases the release of dopamine and glutamate in rat mPFC slices[2]. Animal Model: Adult male Sprague-Dawley rats[2] Dosage: 1-10 mg/kg Administration: Intraperitoneal injection Result: Inhibited avoidance response at concentrations of 1, 3 and 10 mg/kg after 20 minutes. Promoted NMDA and AMPA-sensitive currents, also significantly increased dopamine and glutamate release at 10 mg/kg in mPFC cone cells of rat. |
| References |
[3]. Lumateperone |
| Density | 1.3±0.0 g/cm3 |
|---|---|
| Boiling Point | 556.4±0.0 °C at 760 mmHg |
| Molecular Formula | C24H28FN3O |
| Molecular Weight | 393.497 |
| Flash Point | 290.3±0.0 °C |
| Exact Mass | 393.221649 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±0.0 mmHg at 25°C |
| Index of Refraction | 1.646 |