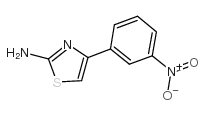
199666-03-0
| Name | 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzenesulfonamide |
|---|---|
| Synonyms |
Ro 61-8048
3,4-Dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzenesulfonamide |
| Description | Ro 61-8048 is a potent and selective inhibitor of kynurenine hydroxylase with IC50 of 37 nM.IC50 value: 37 nM [1]Target: kynurenine hydroxylase inhibitorin vitro:in vivo: Ro 61-8048 blocked rat and gerbil kynurenine 3-hydroxylase after oral administration, with ED50's in the 3-5 mumol/kg range in gerbil brain. In a microdialysis experiment in rats, 16 dose dependently increased kynurenic acid concentration in the extracellular hippocampal fluid. A dose of 100 mumol/kg po led to a 7.5-fold increase in kynurenic acid outflow [1]. A significant reduction in infarct volumes also was found when the kynurenine hydroxylase inhibitors were given to rats after permanent middle cerebral artery occlusion (from 207+/-111 mm3 in vehicle-treated rats to 82+/-18 and to 62+/-57 mm3 in rats treated with mNBA, 400 mg/kg intraperitoneally, or with Ro 61-8048, 40 mg/kg intraperitoneally, respectively) [2]. intrastriatal injections of Ro 61-8048 (60-80 microg/hemisphere) significantly reduced the severity of dystonia in dt(sz) hamsters [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 627.4±65.0 °C at 760 mmHg |
| Molecular Formula | C17H15N3O6S2 |
| Molecular Weight | 421.448 |
| Flash Point | 333.3±34.3 °C |
| Exact Mass | 421.040222 |
| PSA | 159.96000 |
| LogP | 3.38 |
| Appearance | light yellow solid |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.641 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
|
~64% 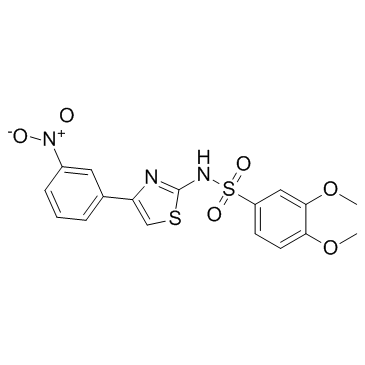
199666-03-0 |
| Literature: J. DAVID GLADSTONE INSTITUTES; UNIVERSITY OF MARYLAND Patent: WO2008/22281 A1, 2008 ; Location in patent: Page/Page column 59 ; WO 2008/022281 A2 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |