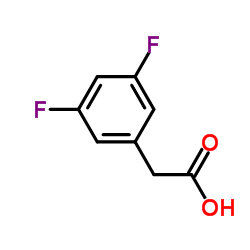
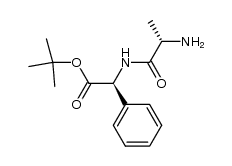
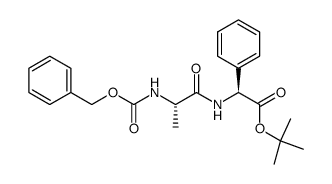
208255-80-5
| Name | tert-butyl (2S)-2-[[(2S)-2-[[2-(3,5-difluorophenyl)acetyl]amino]propanoyl]amino]-2-phenylacetate |
|---|---|
| Synonyms |
GSIIX
(3,5-Difluorophenylacetyl)-L-alanyl-L-2-phenylglycine tert-Butyl Ester 2-Methyl-2-propanyl (2S)-({N-[(3,5-difluorophenyl)acetyl]-L-alanyl}amino)(phenyl)acetate GSI-IX LY-374973 N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (3,5-Difluorophenylacetyl)-Ala-Phg-OtBu tert-butyl (2S)-({N-[(3,5-difluorophenyl)acetyl]-L-alanyl}amino)(phenyl)ethanoate DAPT (GSI-IX) N-[N-(3,5-difluorophenylacetyl)-L-alanyl]-(S)-phenylglycine t-butyl ester dapt |
| Description | DAPT is a γ-secretase inhibitor with IC50s of 115 and 200 nM for total Aβ and Aβ42, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 115 nM (Aβ), 200 nM (Aβ42)[5] |
| In Vitro | DAPT inhibits Aβ production over 90%, effects only a modest reduction in APPβ in the culture media. Although APPβ is reduced by about 30% by DAPT treatment, this effect is not concentration-dependent and is reversed by the removal of DAPT[1]. CNE-2 cells are treated with increasing concentrations of DAPT (0, 25, 50 and 75 μM), and the γ-secretase-generated Notch 1 fragment Val1744-NICD is decreased after 48 h in a dose-dependent manner (P<0.01). The activation of γ-secretase is almost completely inhibited by DAPT at the concentration of 50 μM[2]. |
| In Vivo | DAPT is administered to PDAPP mice (100 mg/kg s.c.) and the levels of DAPT and Aβ are examined in the brain cortex. Peak DAPT levels of 490 ng/g are achieved in the brain 3 h after treatment, and levels greater than 100 ng/g (~200 nM) are sustained throughout the first 18 h. These brain concentrations of DAPT are in excess of its IC50 for lowering Aβ in neuronal cultures (115 nM), and results in a robust and sustains pharmacodynamic effect[1]. DAPT protects brain against cerebral ischemia by down-regulating the expression of Notch 1 and Nuclear factor kappa B in rats. Western blot analyses also show a significant decrease of Notch 1 and NF-κB expression in DAPT (0.03 mg/kg) group (P<0.05 vs. MCAO group)[3]. |
| Cell Assay | Human embryonic kidney cells, transfected with the gene for APP751 (HEK 293) are used for routine Aβ reduction assays. Cells are plated in 96-well plates and allowed to adhere overnight in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum. Cells are pre-treated for 2 h at 37°C with DAPT (0, 0.4, 2, 10, 50 and 250 nM), media are aspirated off and fresh compound solutions applied. After an additional 2-h treatment period, conditioned media are drawn off and analyzed by a sandwich ELISA (266-3D6) specific for total Aβ. Reduction of Aβ production is measured relative to control cells treated with 0.1% DMSO and expressed as a percentage inhibition. Data from at least six doses in duplicate are fitted to a four-parameter logistical model using XLfit software in order to determine potency[1]. |
| Animal Admin | Mice[1] The three- to four-month-old heterozygous PDAPP transgenic mice overexpressing the APPV717F mutant form of the amyloid precursor protein. Each treatment group (n=10) consists of equal numbers of age-matched male and female animals that are fasted overnight prior to treatment. Both treatment and control groups are dosed at a volume of 10 mL/kg with DAPT or vehicle alone. Tissues are processed and all Aβ and APP measurements are made. After removal of the brain, the cortex from one hemisphere is homogenized, extracted with 5 M guanidine, 50 mM Tris-pH 8.0, centrifuged, and the supernatant is used for Aβ measurements. Cortex from the other hemisphere is snap frozen for analysis of compound levels. Aβ levels are expressed as ng/g of wet tissue weight, and percentage reductions are calculated relative to the mean Aβ level of tissue from vehicle-treated control animals. Data are analyzed with Mann-Whitney non-parametric statistics to assess significance. Rats[3] Male Sprague-Dawley rats (260-290 g) are used. DAPT solution is stereotactically injected into the lateral cerebral ventricle (LV) immediately after MCAO. The stereotactic injections into the LVs are performed at coordinates −0.8 mm anteroposterior, ±1.5 mm mediolateral and −4.5 mm dorsoventral from the bregma. 30 rats are randomly assigned to three operating groups (10 rats in each group): sham-operated group that receive equal volume of PBS without MCAO operation; MCAO group that receive equal volume PBS after MCAO (MCAO); and DAPT group that receive DAPT as 0.03 mg/kg after MCAO. 24 h after operation the first neurological function is assessed and then 48 h after operation the second neurological function is assessed. Meanwhile, brain water content and infarction volume are measured and compared among different groups. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 612.2±55.0 °C at 760 mmHg |
| Molecular Formula | C23H26F2N2O4 |
| Molecular Weight | 432.460 |
| Flash Point | 324.1±31.5 °C |
| Exact Mass | 432.186066 |
| PSA | 84.50000 |
| LogP | 3.98 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.535 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ~18 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~85% 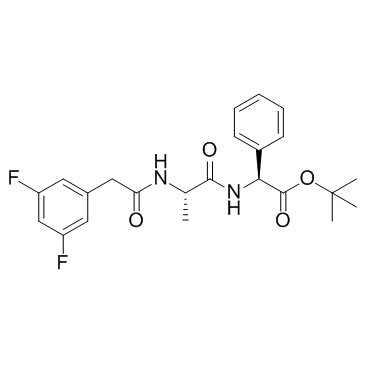
208255-80-5 |
| Literature: Pietrancosta, Nicolas; Quelever, Gilles; Laras, Younes; Garino, Cedrik; Burlet, Stephane; Kraus, Jean Louis Australian Journal of Chemistry, 2005 , vol. 58, # 8 p. 585 - 594 |
|
~% 
208255-80-5 |
| Literature: Australian Journal of Chemistry, , vol. 58, # 8 p. 585 - 594 |
|
~% 
208255-80-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 14, # 8 p. 1983 - 1985 |
|
~% 
208255-80-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 14, # 8 p. 1983 - 1985 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |